Anyone with a professional interest in roasting coffee, or baking bread has heard of the Maillard reaction. But the related reaction called Strecker degradation, is much less well-known and it has a crucial role to play in coffee roasting. In this post, we’re going to explore this under-appreciated reaction in depth, so be sure to have your lab coats and safety goggles at the ready.
A while back we wrote an introduction to the Maillard reactions and their role in roasting. It’s reactions with an ‘s’ because they are a complex web of chemical reactions that result from sugar and protein being heated together. The Maillard reactions contribute a lot to the delicious aroma and colour of many different foods and drinks that ‘go brown’ as they cook, or dry out. Strecker degradation is often considered to be a subset of the Maillard reactions but with coffee in particular, it has a very specific and important role to play. It contributes to much of the characteristic aroma of fresh coffee, and it creates nearly all of the CO2 trapped in your beans. That means Strecker degradation is the main cause of the second crack — we strongly recommend you don’t roast into the second crack. Most intriguing of all, the amount of Strecker compounds in your coffee can be affected by changing how it’s roasted, how it’s brewed, and how it’s stored.
The Chemistry of Strecker Degradation
Strecker degradation was first identified by the German chemist Adolph Strecker in 1862. The reaction converts amino acids, the building blocks of protein, into volatile aroma compounds called aldehydes. Aldehydes typically have distinctive odours, and many of them can be perceived even at very low concentrations, making them an important part of the complex aroma of fresh coffee.
Strecker degradation is a two step reaction: the amino acid is oxidised with the help of another compound (the oxidant), and the resulting molecule then breaks down into an aldehyde, giving off ammonia and carbon dioxide in the process.
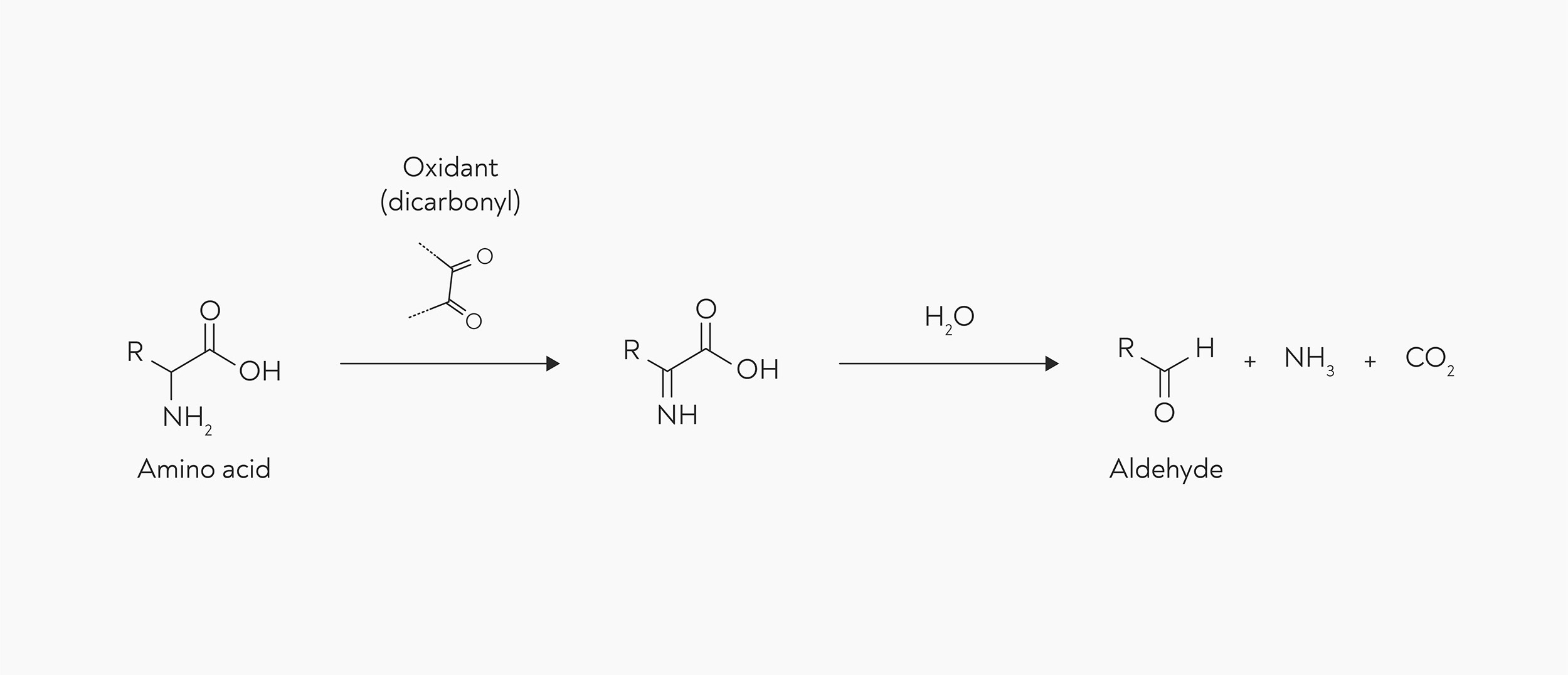 Strecker degradation. An amino acid is converted into an aldehyde with the help of an oxidant molecule. Whatever side chain (R) the amino acid had will form the base of the aldehyde.
Strecker degradation. An amino acid is converted into an aldehyde with the help of an oxidant molecule. Whatever side chain (R) the amino acid had will form the base of the aldehyde.
The oxidant is usually a dicarbonyl compound — a molecule with two oxygen atoms joined by double bonds to neighbouring carbon atoms. The Maillard reactions are a potent source of this kind of molecule, which is why Strecker degradation is closely linked to the Maillard reactions.
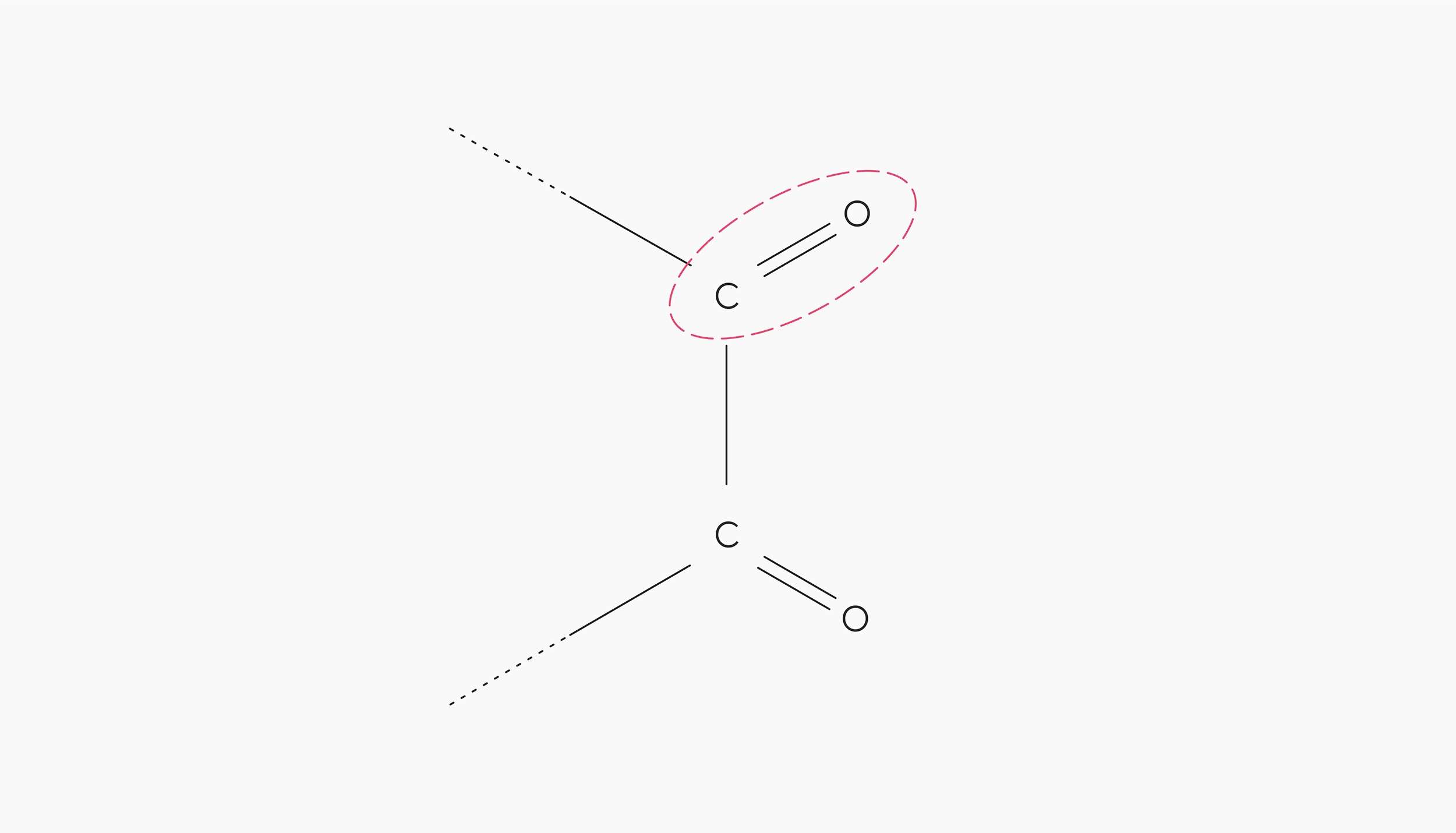 A dicarbonyl molecule has two carbonyl groups next to each other. The carbonyl group (circled in red) consists of an oxygen joined to the carbon chain with a double bond.
A dicarbonyl molecule has two carbonyl groups next to each other. The carbonyl group (circled in red) consists of an oxygen joined to the carbon chain with a double bond.
The resulting aldehyde can go on to form many other compounds, including melanoidins and some of coffee’s most important aroma compounds.
What do Strecker Aldehydes Smell Like?
Aldehydes in general are highly aromatic and widely used in perfumes or flavourings for their floral or fruity aromas. Aldehydes can cover the full spectrum of coffee aromas — anything from floral, grassy, and fruity to nutty, malty, roasted, and earthy. The aldehydes formed in Strecker degradation are a crucial part of the aroma of fresh coffee and can be used as an indicator of coffee freshness (Buffo and Cardelli-Freire 2004).
Examples of Strecker aldehydes found in coffee include phenylacetaldehyde, which has a floral or honey-like aroma, and methylbutanal, which is generally perceived as malty or chocolatey (Poisson et al 2020). They typically have low taste thresholds — meaning that you only need a tiny amount of the aldehyde to be able to smell them — which makes them an important part of the coffee aroma.
Strecker degradation also plays a vital role in the Maillard reactions, creating precursors for all kinds of key coffee aromas, including pyrazines which give freshly roasted coffee much of its characteristic earthy, nutty, and roasted aromas (Toledo et al 2016). Since it is involved in the formation of so many important aroma compounds, Strecker degradation helps to ‘steer’ the Maillard reactions towards creating compounds that contribute aroma, not just colour, to the coffee (Yaylayan 2003). That’s why it’s so important to the flavour of the roasted coffee.
Strecker Degradation Causes Second Crack
Each amino acid that breaks down by Strecker degradation also releases a single molecule of carbon dioxide. Surprisingly, this is enough to account for the vast majority — 80% or more — of the CO2 formed inside the coffee bean during roasting (Wang and Lim 2017). During roasting, some of the carbon dioxide formed during roasting is trapped in the coffee cells, gradually increasing the pressure inside.
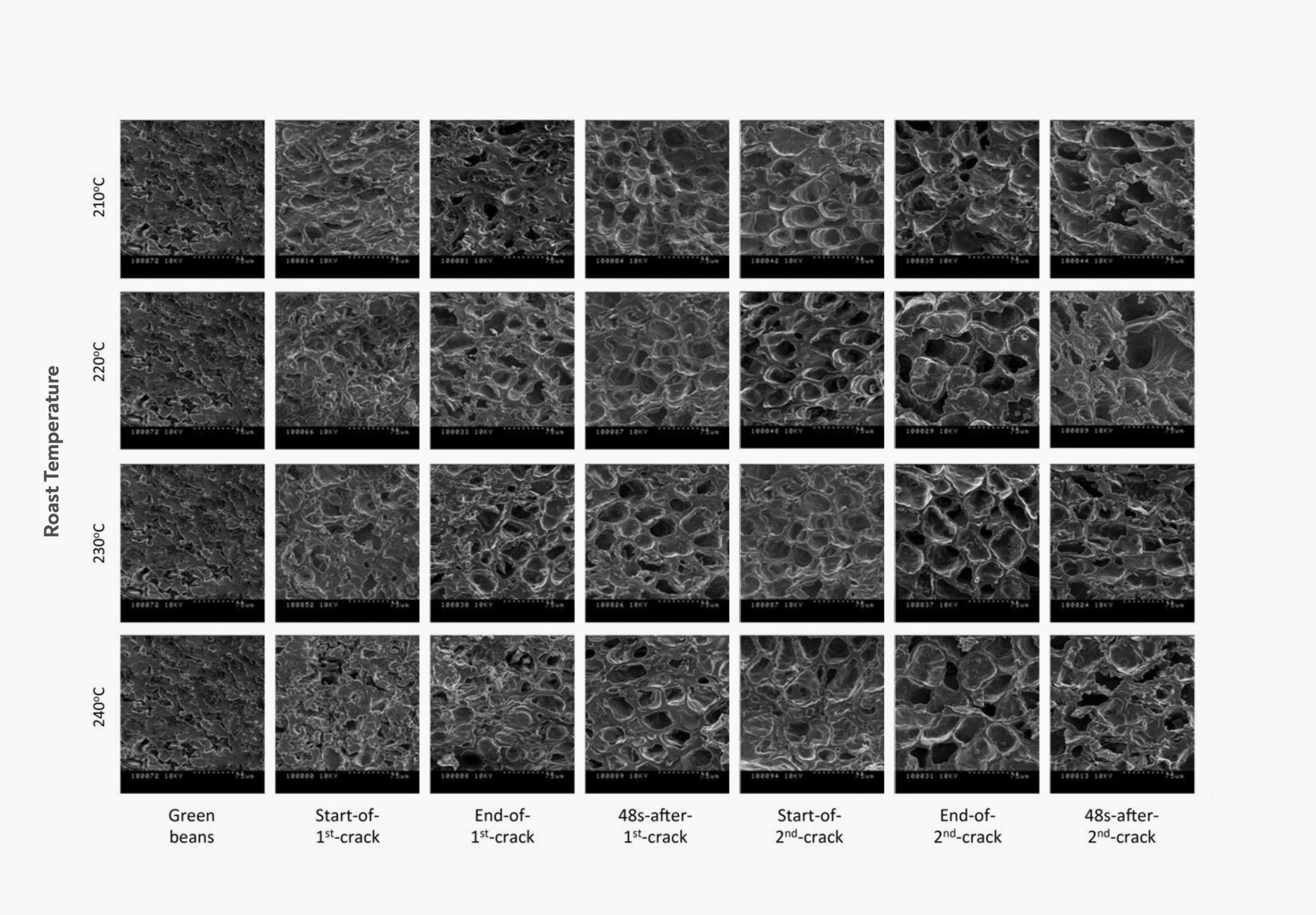 The effect of first and second crack on the structure of a coffee bean. These pictures, taken with an electron microscope, show how the cells swell and rupture during second crack. Taken from Wang (2012).
The effect of first and second crack on the structure of a coffee bean. These pictures, taken with an electron microscope, show how the cells swell and rupture during second crack. Taken from Wang (2012).
First crack is caused by water evaporating to steam, which builds up pressure inside the bean until it breaks open the bean structure. Second crack is a similar process that happens when the pressure of carbon dioxide inside the individual cells of a coffee becomes high enough to burst them open. Since Strecker degradation creates most of the CO2 in roasted coffee, it is also the main driver behind the onset of second crack (Lyman et al 2003).
How Does Roasting Affect Strecker Degradation?
Since Strecker degradation relies on Maillard compounds acting as the oxidants, it’s often assumed to mainly take place later in the roast, after the bean temperature reaches 160 °C (Lyman et al 2003). However, Strecker aldehydes tend to break down or evaporate at high temperatures as well — so while they might be being formed at a faster rate at high temperatures, they are being lost from the bean just as quickly.
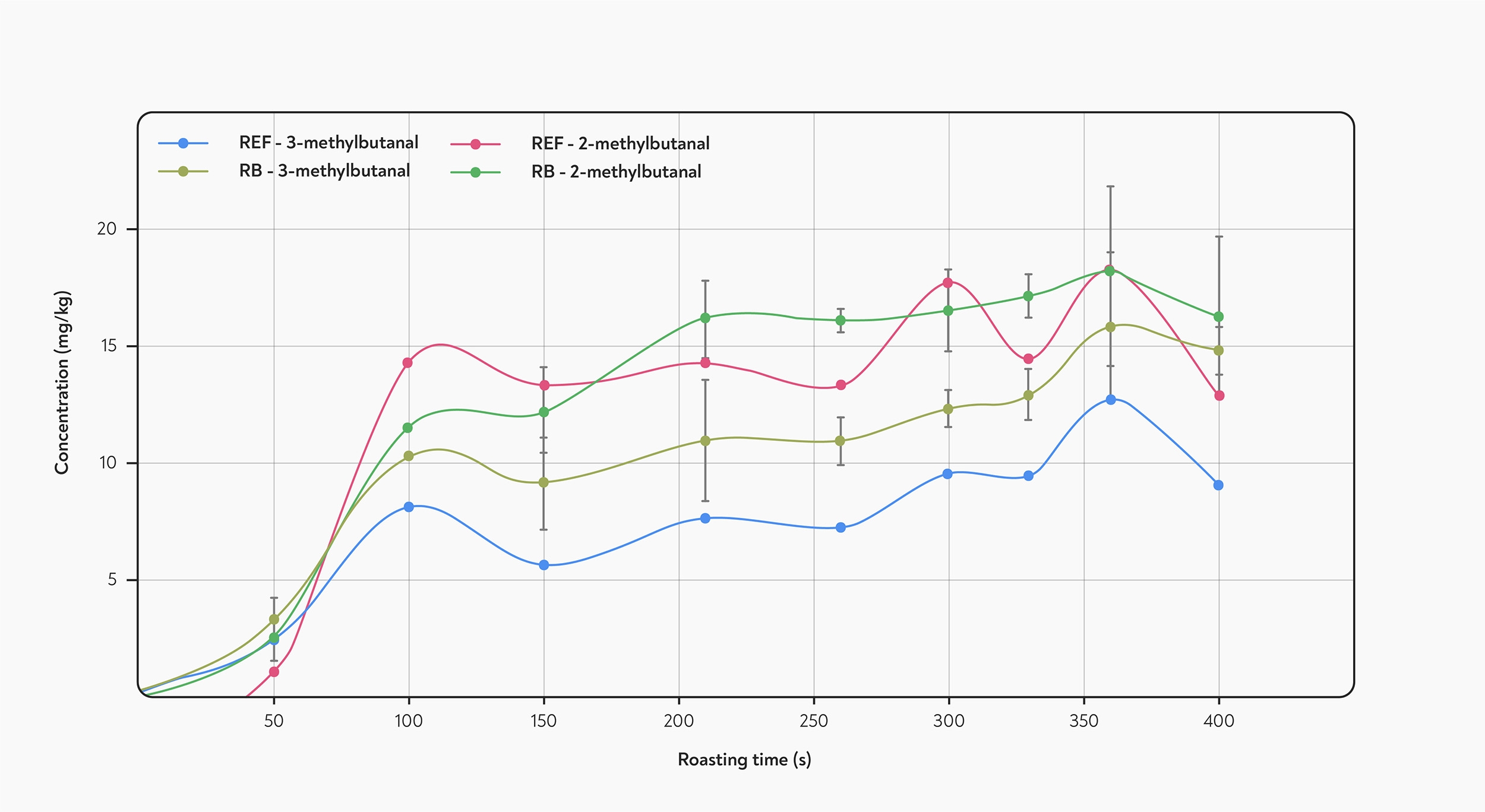 Formation of Strecker aldehydes during roasting. The concentration of the compounds peaks at 100 seconds into the roast, and then plateaus. REF is the reference coffee, while RB is a model roasting system. Taken from Poisson et al (2020).
Formation of Strecker aldehydes during roasting. The concentration of the compounds peaks at 100 seconds into the roast, and then plateaus. REF is the reference coffee, while RB is a model roasting system. Taken from Poisson et al (2020).
Experiments that track the chemicals formed during roasting in real time have mostly found that the concentration of Strecker aldehydes increases rapidly and reaches a peak early during the roast — typically somewhere within the first two minutes (Poisson et al 2020). After this point, the amount of Strecker aldehydes tends to plateau or starts to decrease, as the rate of loss catches up with the rate of formation. Because they break down at higher temperatures, the amount of Strecker aldehydes in coffee is highest in light or medium roasts (Kim et al 2018).
Roasting with a high charge temperature increases the formation of Strecker aldehydes at the start of the roast. For a given roast colour, roasting faster, at a higher temperature, results in a higher concentration of Strecker aldehydes in the roasted coffee (Baggenstoss et al 2008).
Another experiment with excessively fast roasting found that there were some roast compounds, including aldehydes, present in fast-roasted coffee that weren’t found in the standard roast. The authors speculate that these compounds could be responsible for the pungent, grassy aroma of the resulting coffee (Lyman et al 2003). This could imply that some of the ‘green’ and grassy aromas of underdeveloped coffee are formed during roasting, rather than being remnants of the green bean aroma.
As well as roasting, your choice of green beans and even brew method you use affects the amount of Strecker compounds to be found in the final brewed coffee. Strecker aldehydes and pyrazines are found in higher concentrations in coffee made from robusta, and the relative proportion of these compounds in the aroma is higher in espresso than in filter coffee (Maetzu et al 2001).
Strecker Degradation and Antioxidants
While the Maillard reaction and Strecker degradation are thought of as happening at high temperatures, both reactions take place at room temperature as well — just very slowly. Strecker degradation takes place in green coffee during storage (Holscher and Steinhart 1995), and also contributes to the loss of flavour over time in canned, ready to drink coffee.
Since Strecker degradation relies on an oxidant to trigger the first step in the reaction, antioxidants in coffee, such as chlorogenic acid, can reduce the rate of Strecker degradation, helping to preserve the flavour of canned coffee (Zheng et al 2015).
Chlorogenic acid, and other antioxidants, may also inhibit Strecker degradation during roasting. In one experiment designed to model specific reactions under roasting conditions, researchers roasted a single purified amino acid together with glucose (Wang and Ho 2013). They found that adding antioxidants strongly decreased the amount of Strecker degradation. Whether this has any effect in the roasting of real coffee remains to be seen.
The chemistry of roasting is enormously complex, and the roasting community doesn’t yet have a strong grasp of how to control the specific chemical reactions that take place during roasting. Strecker degradation, however, has some interesting levers that we can pull — particularly by tweaking the charge temperature and roasting time. Strecker degradation has often been lumped in with the Maillard reactions, but recent research shows that what happens at the start of the roast can affect how the Strecker compounds develop, which in turn affects what flavour compounds are created in the Maillard phase of the roast. It turns out that this lesser-known reaction has the potential to steer the Maillard reactions, rather than simply following in their wake.
Thanks to Five Elephant for the header photo in this article.



would be interesting to understand how best to maintain highest levels of Strecker aldehydes without destroying or limiting them with the continuing roast to further develop flavors. obviously, knowing what to control means choosing between flavor assets and aromatics for the finished cup.