by BH Learn
At Barista Hustle, we talk about tannins fairly often. We have posted about how tannins are responsible for astringency, the mouth-drying feeling you get in coffee, and how they may be the culprit behind some of the negative flavours associated with ‘over-extraction’ and channelling. Because astringency is such a key component of over-extraction flavours, and because controlling channelling is so important in achieving high, even extractions, this topic comes up frequently.
And yet, there is some debate, going right back to the 1920s, as to whether coffee contains tannins at all.
Until now, we’ve mostly skirted around this topic, talking about pseudo-tannins or tannin-like molecules as well as tannins. We want to finally address this, and be clear about what molecules we’re referring to when we talk about tannins.
Our position is that there is plenty of evidence that some kind of tannins are present in coffee. In fact, the broadest definition of tannins includes familiar molecules like the chlorogenic acids.
We expect that larger tannins are responsible for much of the negative flavours and mouthfeel associated with channelling and over-extraction. While there’s some evidence that such molecules may exist in coffee, it’s not yet known exactly which molecules are involved. Until we learn more about the specific compounds involved, ‘tannins’ is the most accurate and precise word that we can use.
In the rest of this paper, we’ll explain why.
What are tannins?
Broadly speaking, tannins are polyphenolic biological molecules that bind to and precipitate proteins. Different types of tannins are found all over the plant kingdom, including in tree heartwood and bark, in unripe fruits, and in the leaves of plants such as tea.
Because tannins bind to proteins, they have an astringent effect in foods. The dry feeling of astringency in the mouth results from tannins binding to salivary proteins and interfering with the ability of saliva to lubricate the inside of the mouth.
The word ‘tannin’ comes from the leather industry, where tannins derived from plants were used to ‘tan’ animal skins, making them more durable. One of the main sources of tannins was oak bark, called tann in ancient Celtic languages, but any plant compound that would bind the proteins in animal skin could be used.
Because of the importance of this effect, whether a particular compound is considered a tannin is generally determined by its ability to bind proteins, rather than a specific chemical structure. This means that unlike some categories of chemicals, the definition of what constitutes a tannin is not necessarily rigid or universally agreed on, and different definitions have been proposed over the years (S Quideau 2009).
Most tannins fall into one of three main categories, based on their chemical structure: Hydrolysable tannins, based on gallic acid; condensed tannins, based on flavanol, and the phlorotannins, found in brown algae. However, other types of tannin have been identified and our knowledge of these compounds is always expanding: for example, a group of chemicals called oligostilbenoids were first identified to act as tannins in 1993 (Boralle et al 1993).
In the early twentieth century, some tannin-like compounds were isolated from coffee and called ‘caffetannic acid’, assumed to be a type of tannin like those found in tea. However, ‘caffetannic acid’ turned out to be not a type of tannic acid but instead a mixture of molecules, mainly chlorogenic acids (Ukers 1922). This led Lingle in the Coffee Brewer’s Handbook (1996) to state that the phenolic compounds in coffee were “incorrectly classified as tannins”, based on this early research. However, the fact that this one isolate turned out to be mainly chlorogenic acids does not prove that no tannins are present in coffee at all. Plenty of research testing for tannins in coffee has found that they are present — for example, one paper finds that roasted coffee contains about half as much tannin as tea (Savolainen 1992).
Testing for tannins
Since tannins bind proteins, classification of tannins often depends on whether they show a positive result in various chemical tests that involve testing the ability of the tannin to bind protein. One such test is called the ‘Goldbeater’s Skin Test’, in which the presence of tannin causes a distinctive blue-black colour to appear on a thin layer of cattle intestine. Other tests use different protein sources such as gelatin or albumin as the basis of the test.
However, whether a tannin binds to a protein depends on the protein used in the test (Hagermann 1998), so not all tests work equally well with all tannins. As a result, some molecules are classified as tannins by some tests, but not by others. Compounds that show tannin activity in some tests, but that don’t show a positive result in the Goldbeater’s Skin Test, are called pseudo-tannins. One such compound is chlorogenic acid, which is abundant in coffee.
Despite the name, pseudo-tannins are considered a sub-category of tannin by some sources — so a pseudo-tannin is still a tannin. By this definition, chlorogenic acid itself can be included as a tannin, and indeed it is listed as a tannin in the National Library of Medicine’s PubChem database. However, chlorogenic acid is readily soluble in water, so a significant proportion will be extracted even in a well-extracted brew. When we talk about the importance of tannins in the correct extraction of coffee, we are really talking about larger molecules, that will only dissolve in large amounts if we’ve done something wrong.
Larger tannins
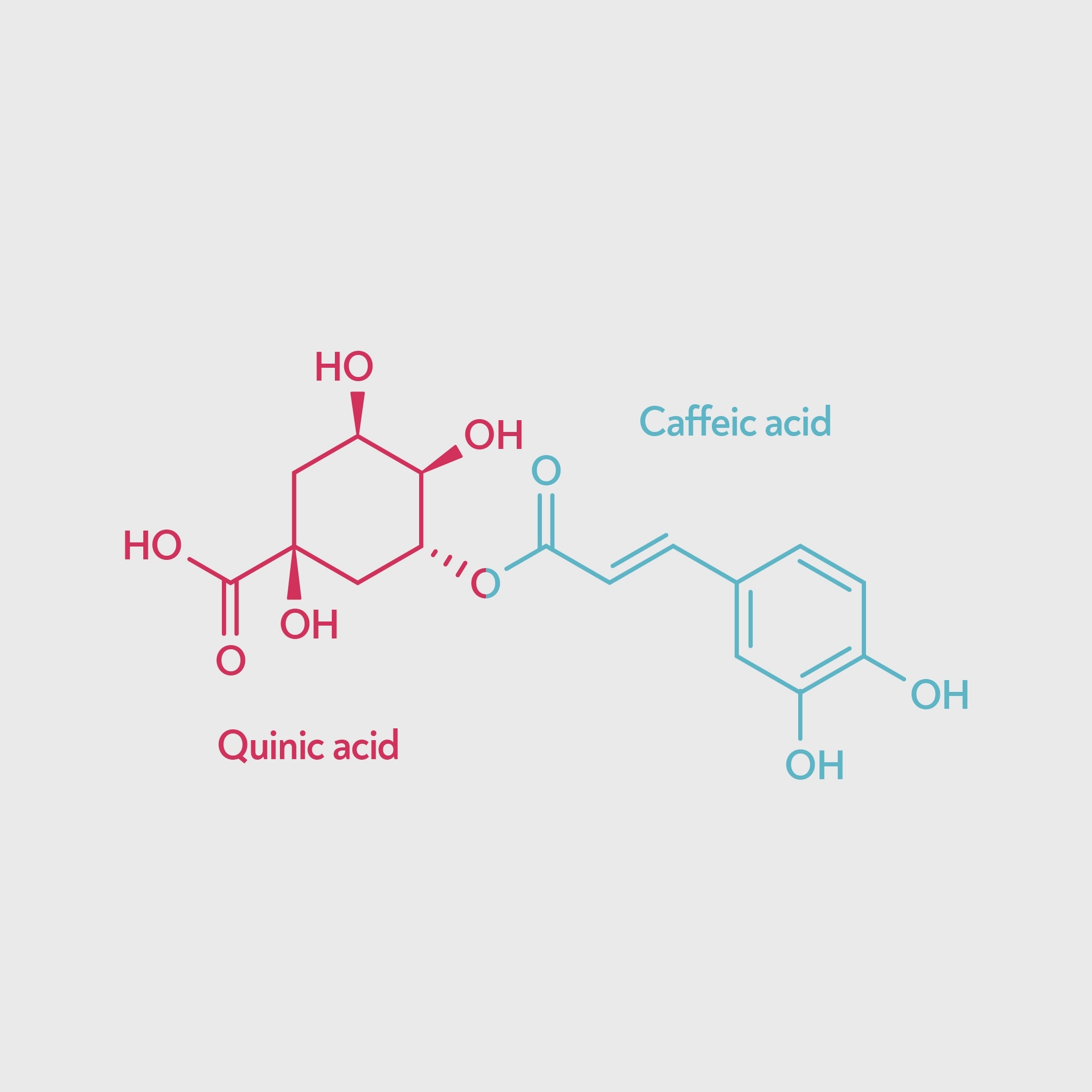
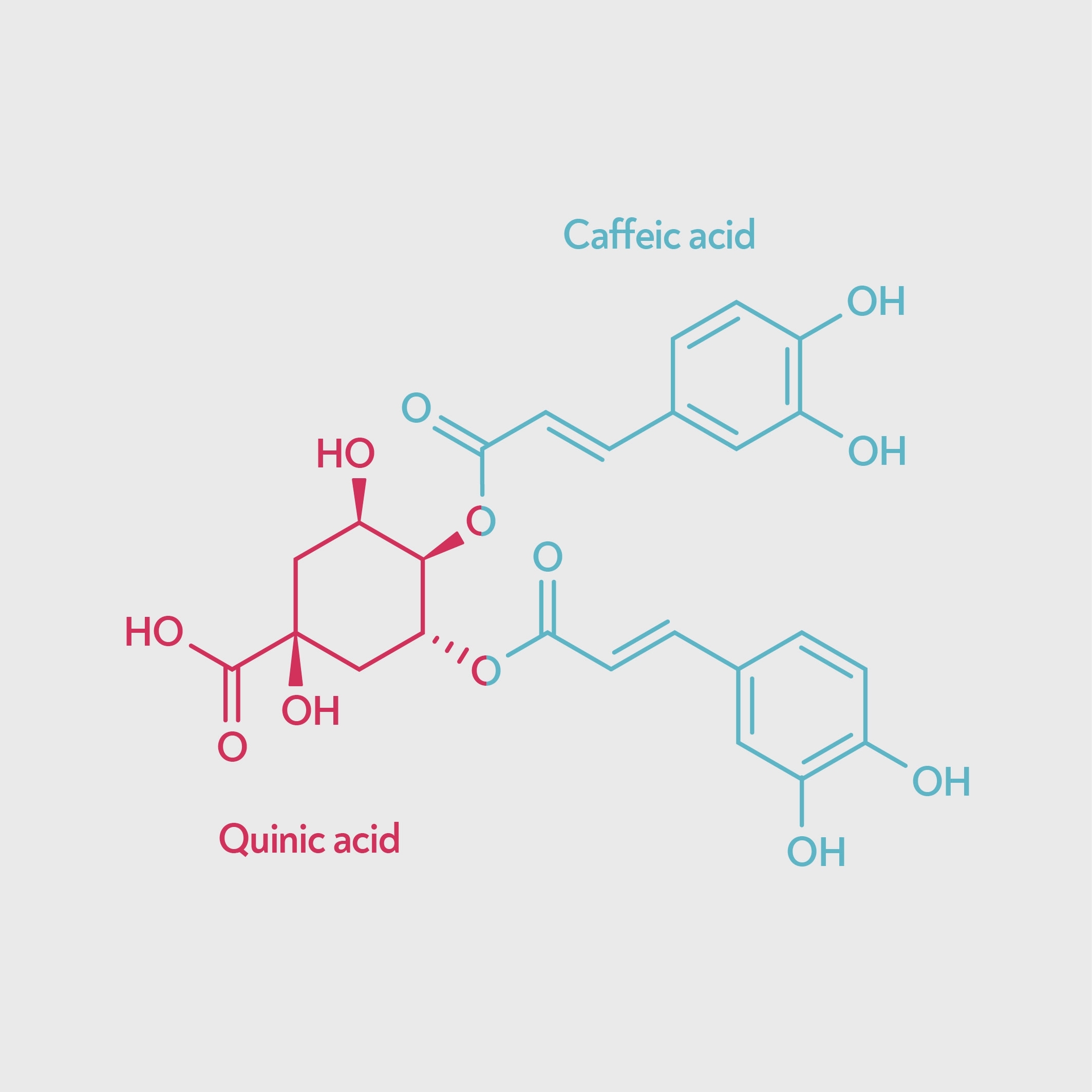
Whether or not chlorogenic acid (CQA) ‘counts’ as a tannin, it’s clear that there are also other, larger molecules responsible for the harsh astringency of a channelled brew. One possible culprit is dichlorogenic acid (di-CQA) — a type of chlorogenic acid that has two caffeic acids attached to the quinic acid instead of one.
Chlorogenic acid (left) and dichlorogenic acid (right). Dichlorogenic acid has two caffeic acid units, so is a larger molecule and binds to proteins more strongly.
Dichlorogenic acid binds to proteins much more strongly than chlorogenic acid (Okuda & Ito 2011) and so has stronger tanning activity, and many sources that don’t count CQA as a tannin do include di-CQA. Dichlorogenic acid may be responsible for the astringency associated with unripe coffee beans, since unripe beans contain a higher proportion of dichlorogenic acid.
More recently, other tannin-like compounds have also been identified in coffee. Using modern techniques that can pin-point the molecular structure of individual molecules, Low et al (2015) found evidence of other types of tannin-like compounds in spent coffee grounds, including catechins, the building blocks of tannins found in tea, as well as the appropriately-named molecule ‘astringin’, found in wine. The way the molecules are extracted for testing makes it hard to definitively say if these building blocks are joined together to form large tannins in the coffee itself, or if they just exist as individual subunits. However, the authors suggest that the larger tannins are likely to be present.
Why call them tannins?
If we’re not sure exactly what type of molecules are causing the astringent effect that we see in coffee, then why do we still generally use the word tannins when talking about problems with brewed coffee?
For us, the reason is that the word ‘tannin’ is mainly used to tell us what the molecule does, rather than what it looks like. We know that some astringency in coffee is caused by molecules that bind to proteins in the way that tannins do, and that astringency is very important in determining the quality of a brew. Using a more general term such as ‘phenolic compounds’ includes lots of molecules that don’t have this effect, and behave quite differently in an extraction.
For this reason, we need a word that specifically describes larger molecules that bind to proteins and cause astringency, and ‘tannin’ fits the bill exactly. This word is widely used this way, in both the coffee industry and in the scientific literature. In fact, having chosen our preferred definition of tannins, to use another word such as ‘phenolic molecules’ is both less accurate and less precise. As always, we are happy to update our position as new information becomes available, or widely accepted definitions change, but as things stand we are quite confident in saying that yes, there are tannins in coffee.


0 Comments