How do ion exchange water filters work?
We all know that hard water is the enemy of good coffee – it can make coffee taste heavy, chalky, and dull, and will wreak havoc with your equipment too.
Ion exchange water filters are the most common method for reducing hardness in water – they’re relatively cheap, and the most basic versions can even be ‘regenerated’ and used again.
A Quick Refresher on Water Hardness
The ‘hardness’ of water is a measure of the concentration of calcium and magnesium minerals in the water. This is divided into ‘temporary hardness’ also known as alkalinity, which refers to the concentrations of calcium and magnesium bicarbonate, and ‘permanent hardness’, which refers to all other calcium and magnesium minerals, such as sulfates and chlorides. Both added together, or the total concentration of calcium and magnesium is referred to as ‘total hardness’.
These minerals have different effects on coffee flavour and on the equipment, so the aim of water treatment is to selectively remove mineral ions, for example, calcium (Ca2+), Magnesium (Mg2+), or bicarbonate (H2C032-) from the water.
Reducing Hardness
The net charge in water must always be neutral – so if you remove positively charged calcium ions, then either an equivalent amount of negatively charged ions (e.g. bicarbonate) must be removed, or they must be replaced with other positively charged ions (e.g. sodium). Ion exchange resins work by replacing the ions we want to remove with other ions of the same charge that don’t contribute to hardness – usually by replacing calcium ions with sodium.
Ion Exchange Resins
A typical water filter contains a long column, filled with beads of a polymer resin. The small beads mean there is a large surface area in contact with the water as it flows past.
In the case of a calcium exchange filter, the resin is made of a polymer that binds positively charged ions. The polymer is designed to bind to calcium and magnesium more strongly than to sodium. Initially, the polymer is saturated with sodium ions, meaning that there are sodium ions at the surface of each bead available to be exchanged.
When hard water flows past this resin, the calcium and magnesium ions will bind to the polymer, displacing the sodium into the water. The water flowing out is then softer but contains more sodium instead.
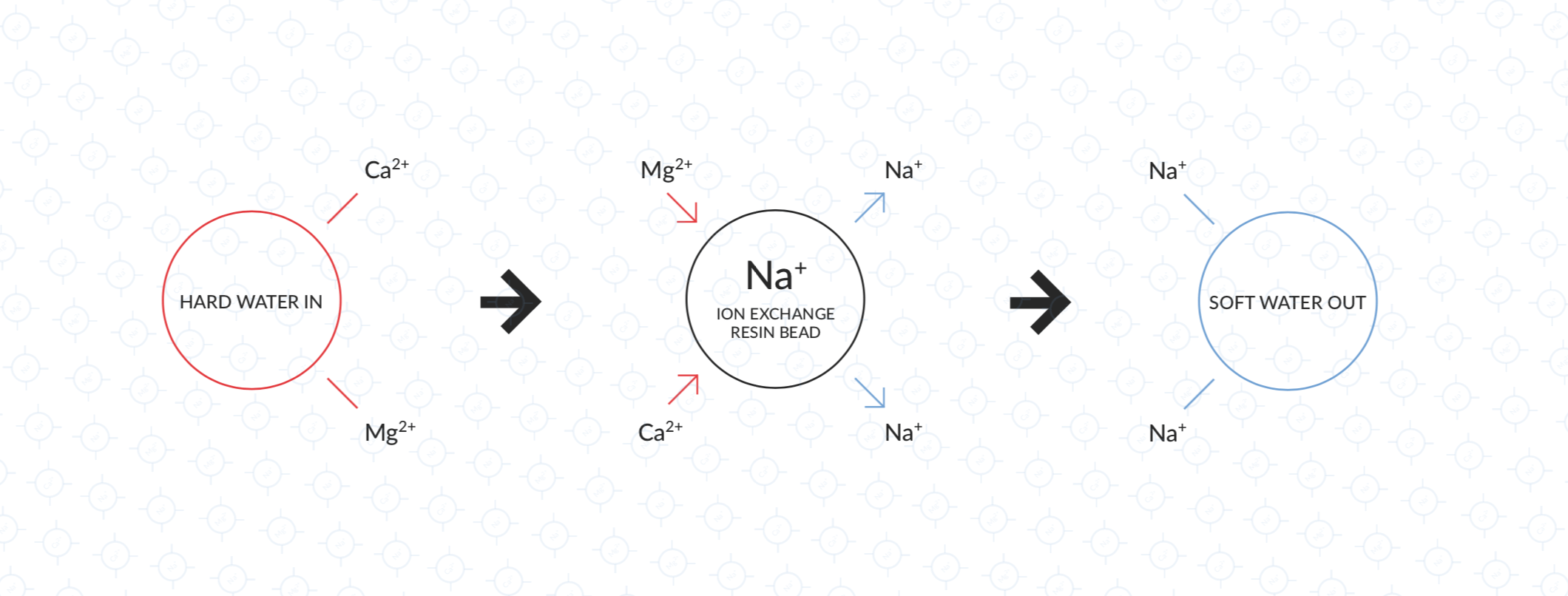
Left: Hard water flows in
Middle: Calcium and Magnesium ions exchange with sodium ions on the resin surface
Right: Soft water with sodium ions added flows out
Once the resin is exhausted, it can be regenerated by passing over a strong salt solution, forcing the sodium to bind to the resin again, so it can be re-used.
Other ions, such as bicarbonates or heavy metals, can be removed in the same way with different kinds of resin. A typical modern water filter will contain several different types of resin, so that it can reduce both the total hardness and the alkalinity. Some ion exchange filters even exist that are designed to increase the hardness, by adding magnesium to the water, to improve coffee flavour.
What Else is in a Filter?
After water passes through the ion exchange resins, it passes through a final filter stage made of activated carbon. This absorbs other contaminants from the water – including chlorine and organic compounds, that affect the taste of the water – but doesn’t change the mineral content.