Brewing temperature affects the flow rate of espresso in unexpected ways, thanks to dissolved gases.
In our most recent instalment of the Advanced Espresso course, we discussed a surprising finding in the scientific literature: that increasing the brew temperature makes espresso shots run more slowly. We set out to test this for ourselves, and discovered that the truth is more subtle: it turns out that changing the temperature has a different effect on flow rate, depending on which part of the shot you are looking at.
In fact, if you increase the brewing temperature, the first part of the shot runs more slowly, but the last part of the shot runs more quickly. This means that in our experiment, the total shot time was about the same, but implies you could get different results, depending on what brew ratio you choose.
This effect is bigger in fresher coffee and in a darker roast, which leads us to suspect that the effect is caused by dissolved gases in the coffee. One possibility is that at high temperatures, more bubbles form in the puck during the early part of the shot. This somehow leads to lower resistance to flow, or more channeling, during the later part of the shot.
Background
First, let’s look at the existing published evidence: Scientific papers on the subject tend to mention that higher temperatures lead to lower flow rates. This counter-intuitive result was first reported by Marino Petracco (2005), who showed a large difference in flow rate when brewing at 4° C compared to 90° C.
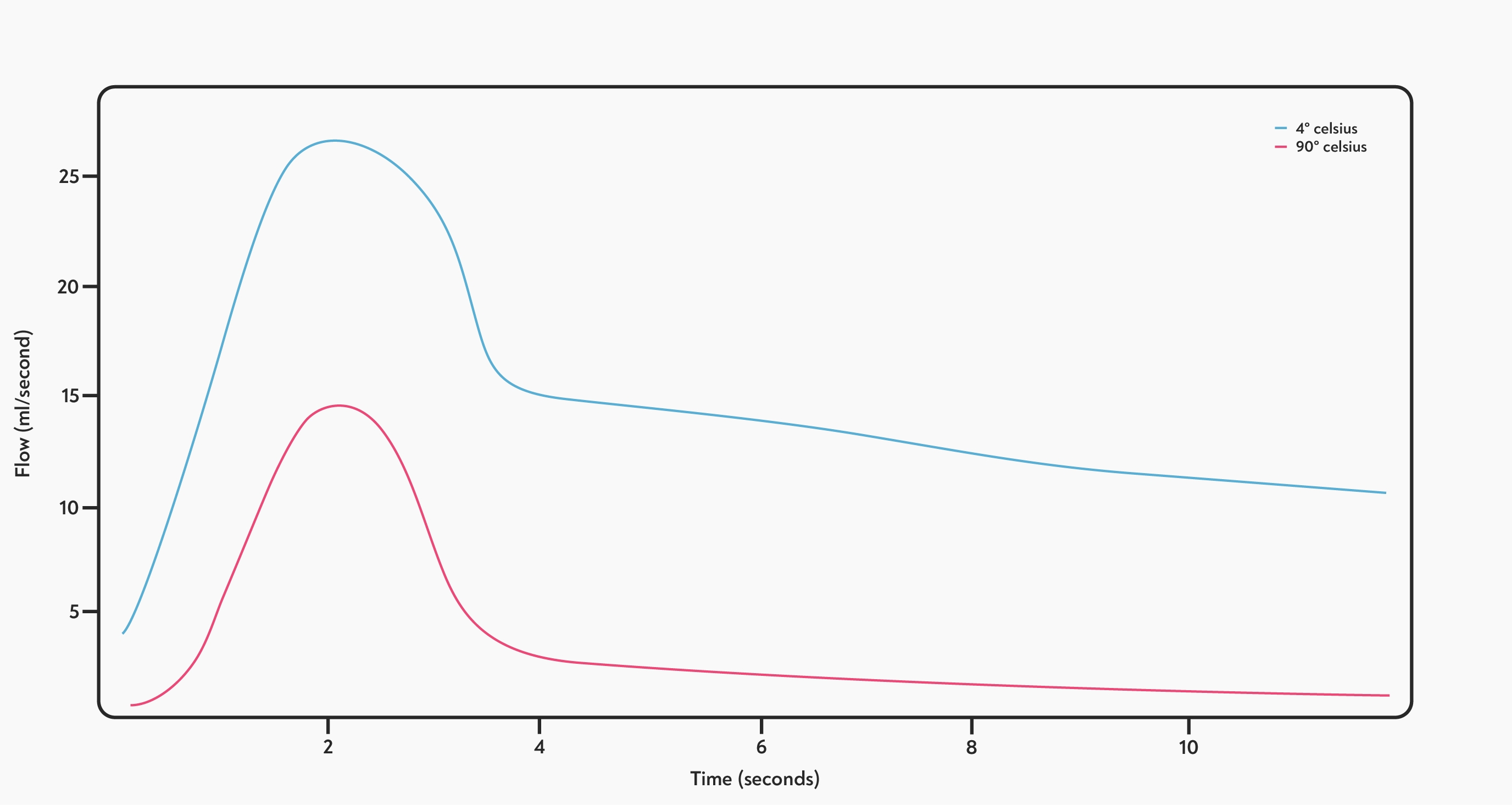 Flow rates of espresso brewed at 4° C and 90° C. Adapted from: A. Illy And R. Viani (Eds), Espresso Coffee: The Science of Quality, p268.
Flow rates of espresso brewed at 4° C and 90° C. Adapted from: A. Illy And R. Viani (Eds), Espresso Coffee: The Science of Quality, p268.
This effect on flow is easily noticeable when dropping the temperature from 90° C to 70° C, but will also affect shots under realistic brewing conditions, Petracco claims. “The effect is slighter, yet significant, at temperatures around the optimal value.” However, the paper that he cites (S. Andueza et al., 2001) doesn’t seem to support this claim.
Meanwhile, the results of experiments by baristas under realistic brewing conditions are contradictory. While one experiment by Monika Fekete supported the idea that temperature could affect flow within a relevant temperature range (90–95° C), another set of experiments by Andre Eiermann, the 2017 Swiss Barista Champion, found that there was no effect on shot time between 90 and 98° C. Baristas’ anecdotal experience seems to vary, suggesting that the effect might be contradictory for different coffees, or different equipment.
To verify for ourselves what was going on, and to find out why different people got different results, we set up an experiment of our own.
The Results
We made shots from three coffees — one three months off roast, a fresh light roast, and a fresh darker roast, taking care to control for factors like the grinder temperature (full experimental details are below). We found that the shot time didn’t change overall with temperature for any of the three coffees.
However, we did see a significant decrease in the time it took for the last 20 g to come out, meaning the shot runs more quickly at the end — this effect was larger in fresh coffee and largest of all in a darker roast.
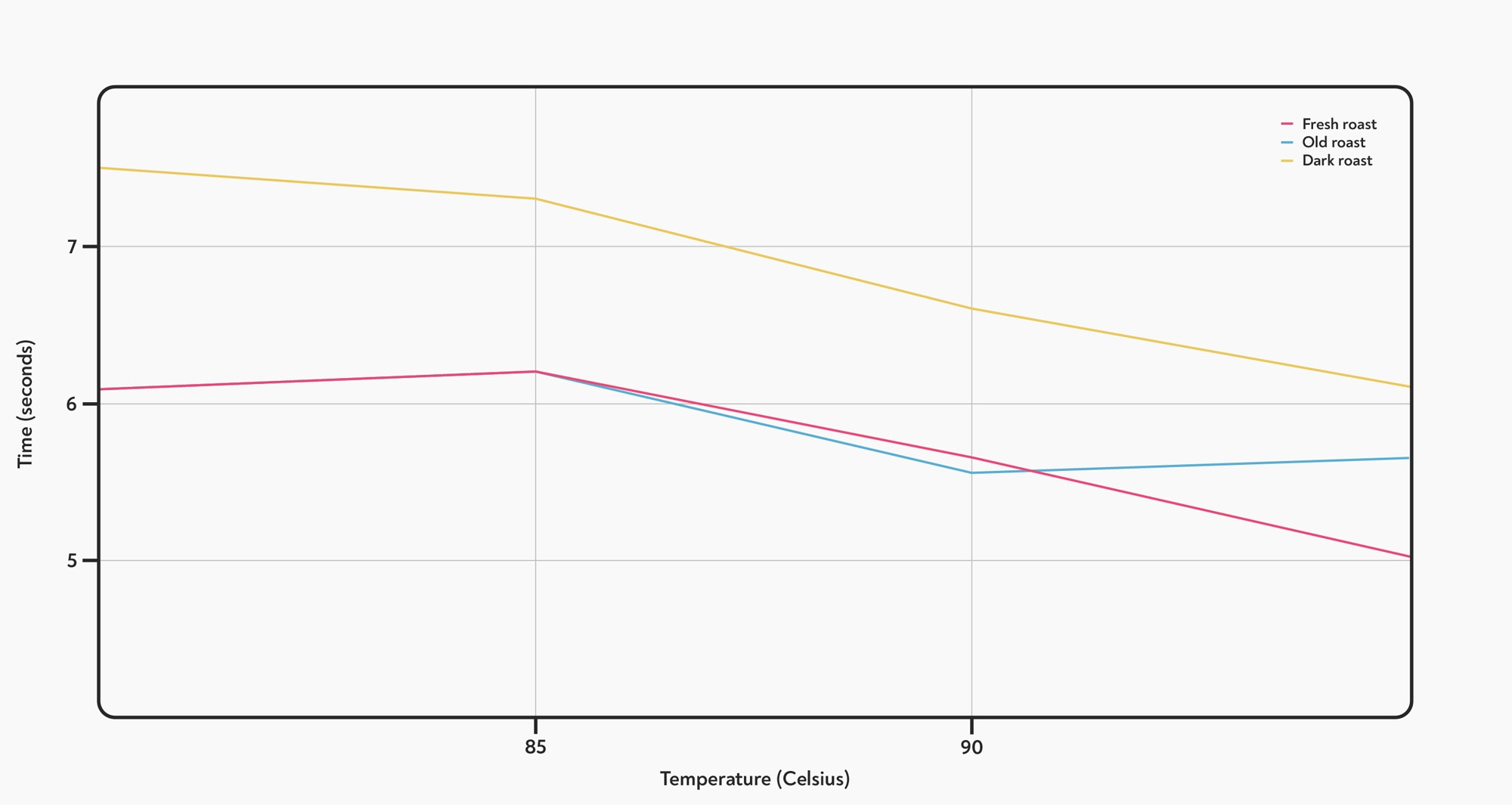 The effect of temperature on the time taken to dispense the last 20 g of each shot. The last part of the shot runs more quickly at higher temperatures. The difference is bigger for the fresher roast, and biggest of all for the dark roast.
The effect of temperature on the time taken to dispense the last 20 g of each shot. The last part of the shot runs more quickly at higher temperatures. The difference is bigger for the fresher roast, and biggest of all for the dark roast.
In fresher roasted coffee, we also saw a small increase in the time it took for the first 20 g to reach the cup — it took on average about 1 second longer at 95° C than at 80° C in freshly roasted coffee.
On the other hand, in coffee that was 3 months off roast, the first part of the shot didn’t slow down noticeably, and the increase in flow at the end was smaller — which suggests that dissolved gases could play a role in this effect.
How Can We Explain This?
One possibility is that this behaviour is caused by gases in the coffee bed. Gases such as CO2 play an important role in espresso extraction. Roasting gases trapped in the coffee grounds are released on contact with water. Some of the gasses dissolve in the water, but some may create bubbles that increase resistance to the flow of water.
At higher temperatures, gases are less soluble in water. It’s likely that this means at higher temperatures, more bubbles form, which would increase the resistance in the bed. This could explain why the first part of the shot gets slowed down, while the bubbles are being released. Once the bubbles have been pushed out of the puck, or enough water has been pumped in to dissolve the remaining gas, this effect stops and the flow increases again.
However, this doesn’t explain why the second half of the flow gets faster, rather than just reverting to the same level. It seems most likely that this is due to increased channeling at higher temperatures — either because the higher extraction at higher temperatures means the puck is starting to break down more quickly, or perhaps because the formation of bubbles disrupts the puck in some way.
The fact that the increase in flow at the end is bigger in both fresher and darker roasted coffee, however, suggests that this is also caused by the dissolved gases — and that the bubbles are somehow causing channels to form later in the extraction. The bubbles might be physically disrupting the puck on a very small scale, but it seems most likely that they’re leaving small dry patches. Since dry coffee is hydrophobic, water will flow around these dry patches instead of through them, leading to channeling.
Another possibility is that the slowed flow at the start gives the bed more time to become fully wetted before the full flow starts, and this reduces fines migration — in a similar way to how a low pressure pre-infusion allows the later part of a shot to flow more quickly.
Is There a Practical Application?
These effects on flow rate are pretty small for the kind of temperatures that you’re likely to be brewing with, so in the most part it won’t play a major role in your espresso. Based on our experiments, the biggest effect that you’d expect to see from a 1° C temperature increase would be to make the second half of your shot flow just one tenth of a second faster.
Even if you make a fairly large change in brewing temperature, the other effects of temperature on brewing are going to have a much bigger effect than the flow. However, this result does tell us something interesting about the role that gases play in espresso extraction. This could be part of the reason that ‘blooming’ in espresso has such a high effect on extraction — giving time for the gases to dissolve or escape removes bubbles and makes the pre-infusion more effective.
The Experiment
For those of you that want the full experimental details and more detailed discussion of some of the results, read on!
The charts get quite complex in the next section so BH writer/researcher, Tom Hopkinson walks us through it in this screen grab.
Protocol
We pulled shots in these experiments at a fixed ratio with an 18-g dose, a 40-g yield and we used temperatures ranging from 80–95° C. We measured the overall shot time and the time taken to reach 20 grams in the cup. To avoid the results being affected by the grinder heating up, we randomised the temperature for each shot.
We used an EK43 on a fixed grind setting, and pulled the shots on a La Marzocco Linea Classic, using the same group head each time. When changing temperature, after the boiler temperature stabilised, we purged the group thoroughly, then allowed 10 minutes to pass, to allow the group temperature to equilibrate. The group was also purged with a fixed volume of water immediately before brewing each shot to bring all parts up to operating temperature.
Results
We started out by testing two coffees: one 3 months off roast, and one 3 days off roast. We made shots with a fixed recipe with 3 different coffees, and measured the time that it took to reach 20 g and 40 g in the cup. As with Eiermann’s experiment, we found no significant difference in the total shot time, or for the time taken to reach 20 g.
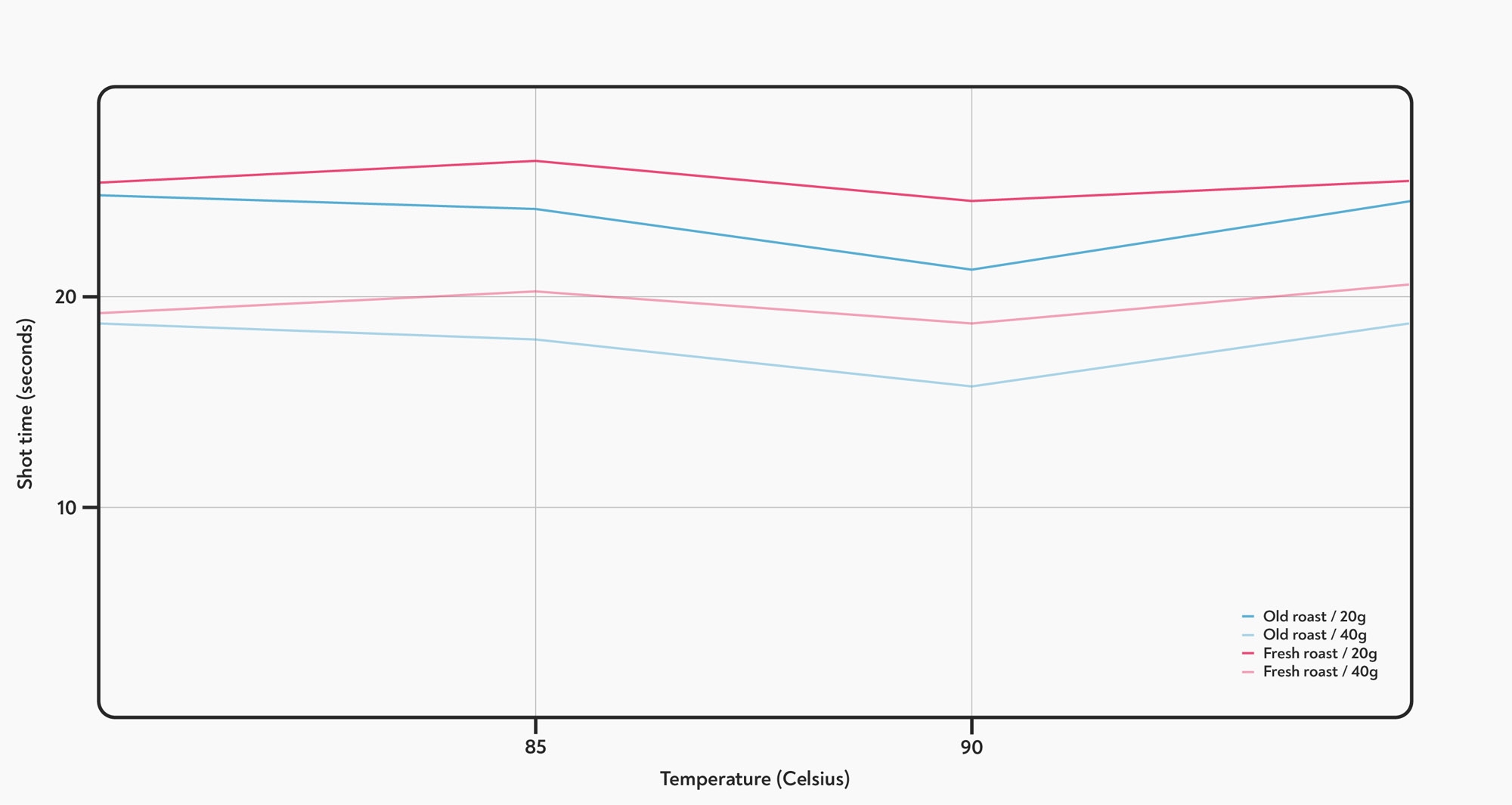 The effect of temperature on the time taken to reach 20-g and 40-g yield. Brewing temperature had no overall effect on shot time.
The effect of temperature on the time taken to reach 20-g and 40-g yield. Brewing temperature had no overall effect on shot time.
However, we did notice something unusual. While the shot times didn’t change overall, the time it took to get from 20 g up to 40 g seemed to get shorter as the temperature went up. The difference between 80° C and 95° C was statistically significant (T-test, p<0.05) for both roasts, although the difference seemed to be larger for the fresh roasted coffee.
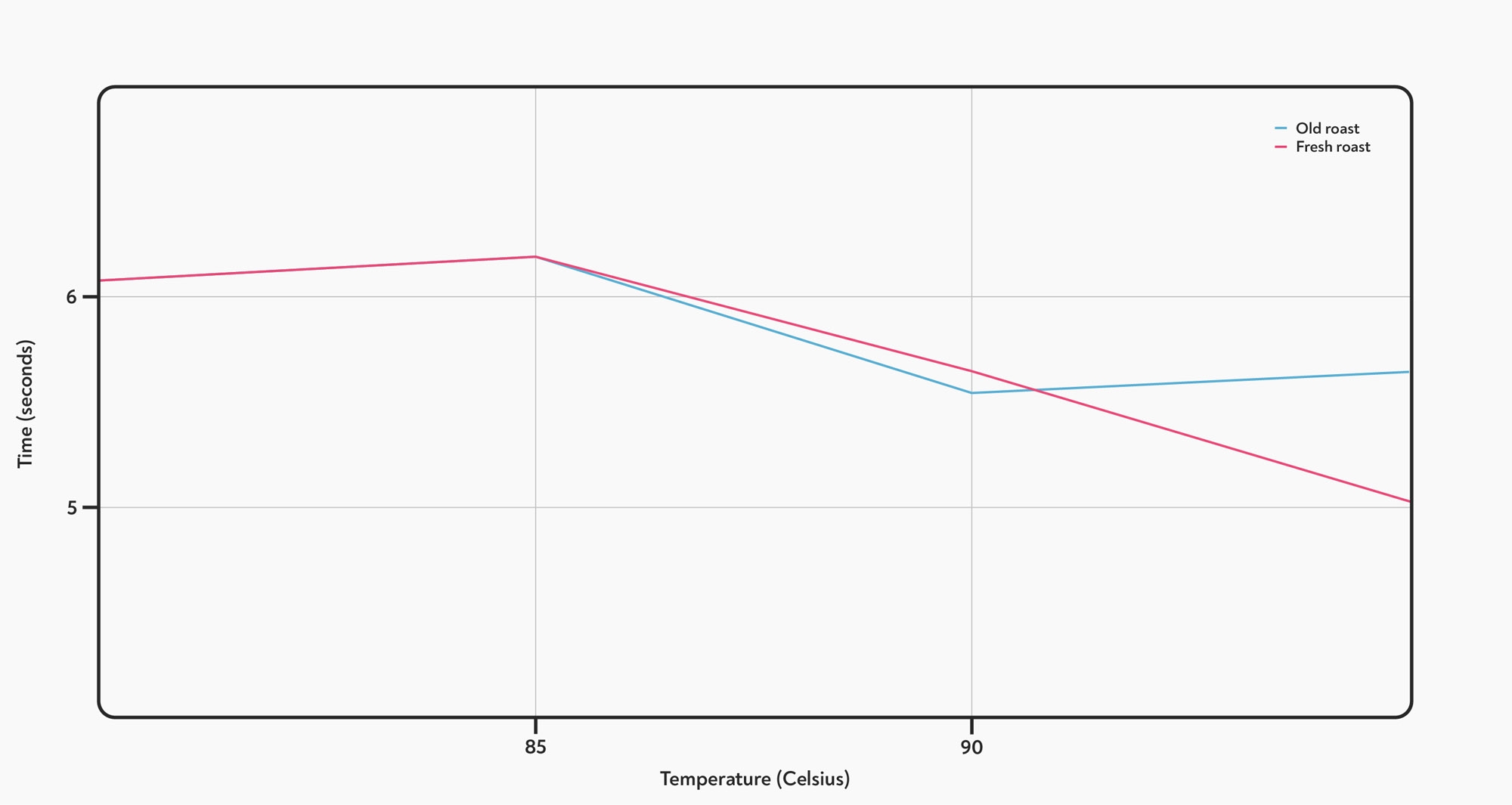 The effect of temperature on the time taken to dispense the last 20 g of each shot. Higher temperatures make the last half of the shot run more quickly.
The effect of temperature on the time taken to dispense the last 20 g of each shot. Higher temperatures make the last half of the shot run more quickly.
We also saw a small increase in the time taken to reach 20 g in the fresh coffee — about 1 second on average between 80° C and 95° C. Because this effect is fairly small however, random variation in shot time makes it hard to visualise the effect. The first part of the shot is responsible for much of the variation in shot time, so changes in overall shot time can obscure the difference in how long it takes to reach 20 g. This variation also means the results are not statistically significant by themselves.
The easiest way to see what’s going on in this part of the shot is by looking at how fast the first part of the shot runs, relative to the total shot time — in other words, the ratio between the time taken to reach 20 g and the time taken to reach 40 g.
We saw that this ratio increased with temperature. In other words, no matter what the actual shot time, higher temperatures seemed to slow down the first part of the shot compared to the second.
Again, the difference between 80° C and 95° C was statistically significant (T-test, p<0.05) for both roasts, and the difference seemed to be larger for the fresh roasted coffee.
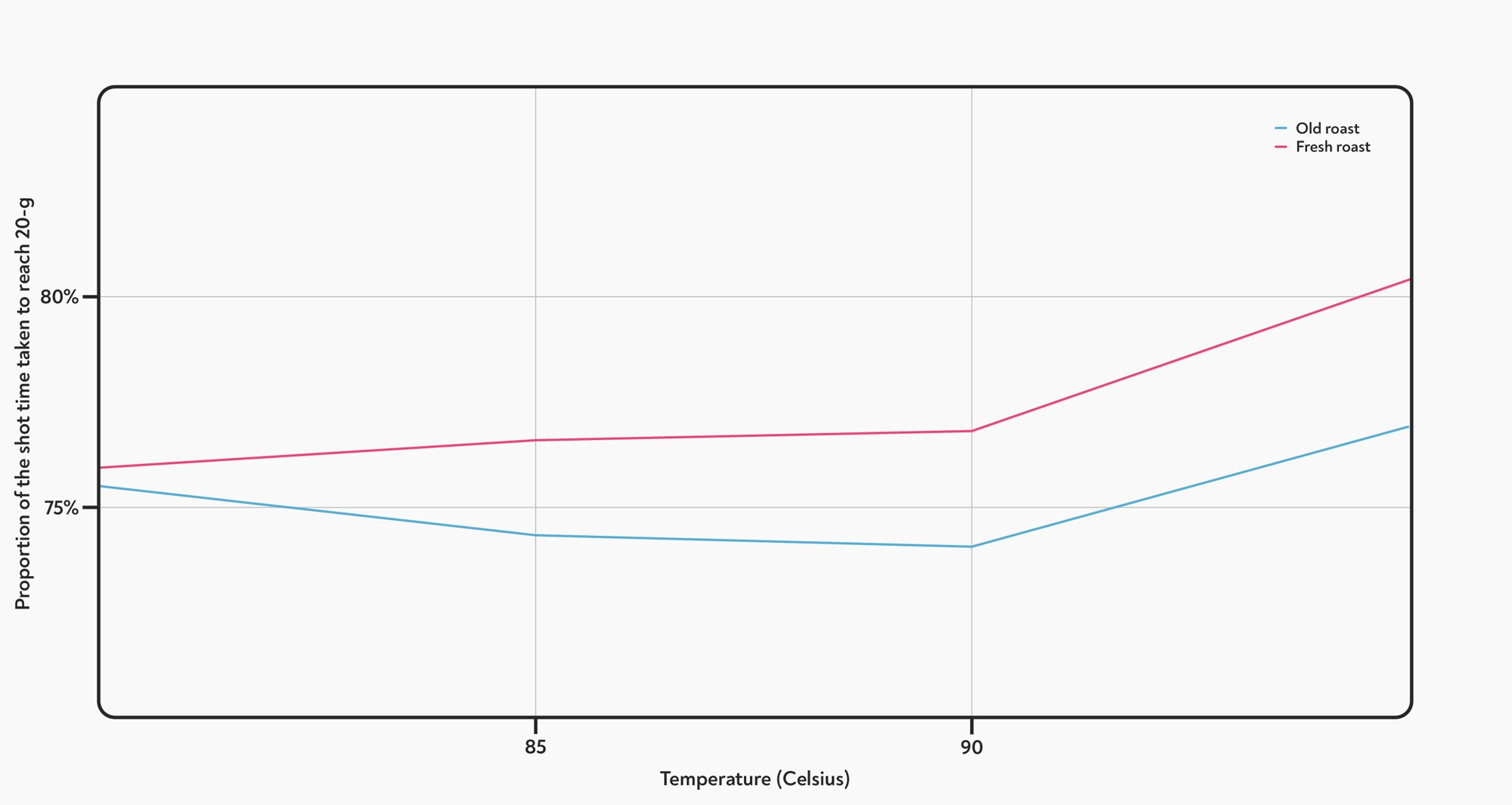 The effect of temperature on the proportion of the total shot time taken to dispense the first 20-g of each shot. Higher temperatures make the first part of the shot run more slowly, when compared to the overall shot time.
The effect of temperature on the proportion of the total shot time taken to dispense the first 20-g of each shot. Higher temperatures make the first part of the shot run more slowly, when compared to the overall shot time.
To confirm that this effect held over a wider temperature range, we then ran a couple of extra shots at 60° C, and found that the trend continued.
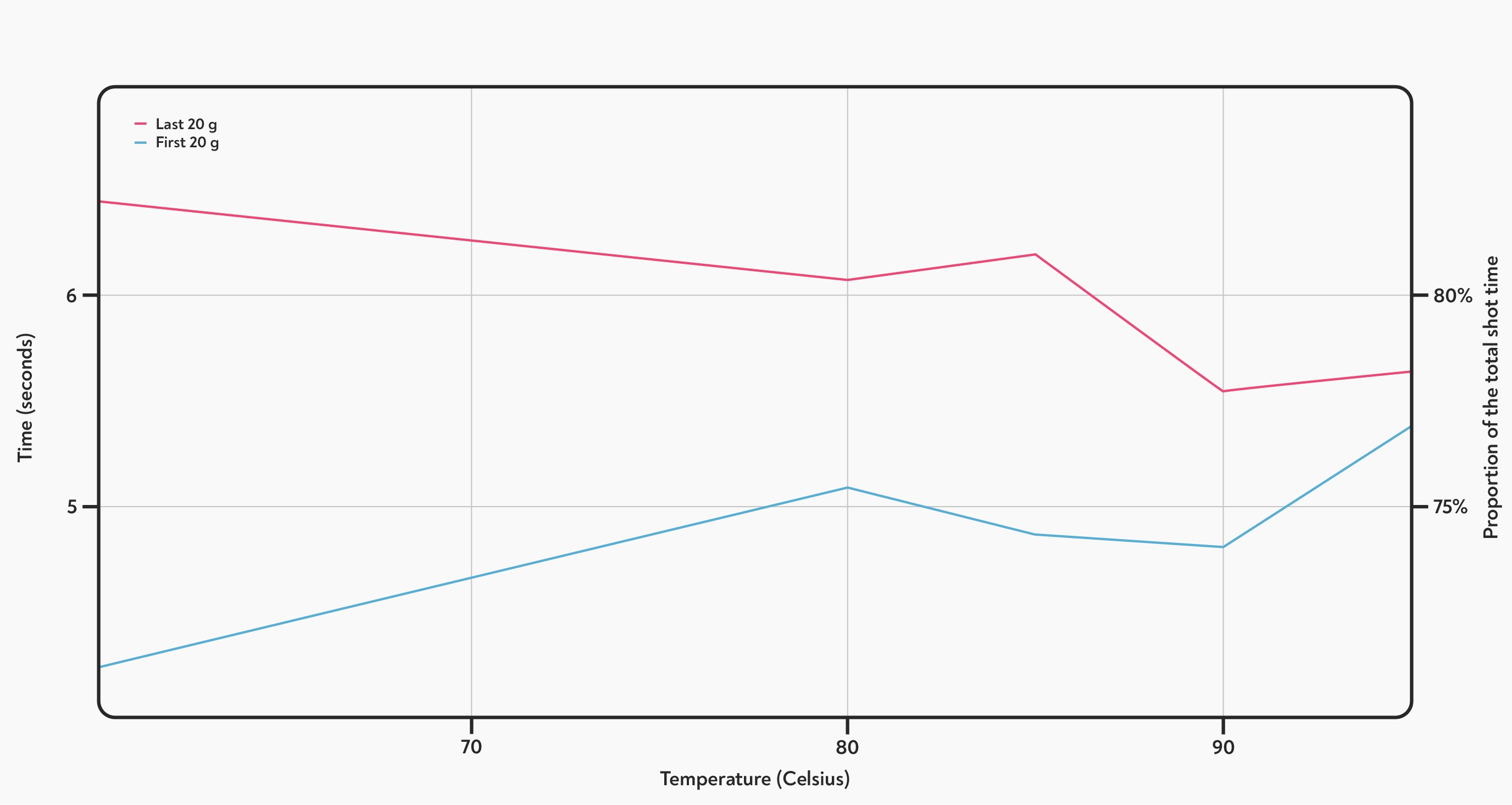 The effect of a bigger temperature change: the red line shows the total time taken to dispense the last 20 g (left axis), and the blue line shows the proportion of time taken to dispense the first 20-g of each shot (on the right hand axis). The trend of slower at the start, faster at the end continues all the way down to a brewing temperature of 60° C.
The effect of a bigger temperature change: the red line shows the total time taken to dispense the last 20 g (left axis), and the blue line shows the proportion of time taken to dispense the first 20-g of each shot (on the right hand axis). The trend of slower at the start, faster at the end continues all the way down to a brewing temperature of 60° C.
At this point, we suspected that the effect might be related to dissolved gases, so we repeated the experiment with a darker roasted coffee that would contain more gas — an espresso blend containing 25% Robusta, 4 days off roast.
This coffee showed what was happening more clearly: the total shot time didn’t change at all between 80 and 95° C (29.8 s vs 29.5 s), but the shots took about 1 second longer to reach 20 g on average (23.4 s vs 22.3 s), although this was also not statistically significant.
The proportion of time taken to reach 20 g increased in a similar way to the fresh roasted coffee, and the last 20 g flowed much more quickly (7.5 s vs 6.1 s) — an even bigger effect than we saw with the fresh light roast. These results were statistically very significant (p<0.005).
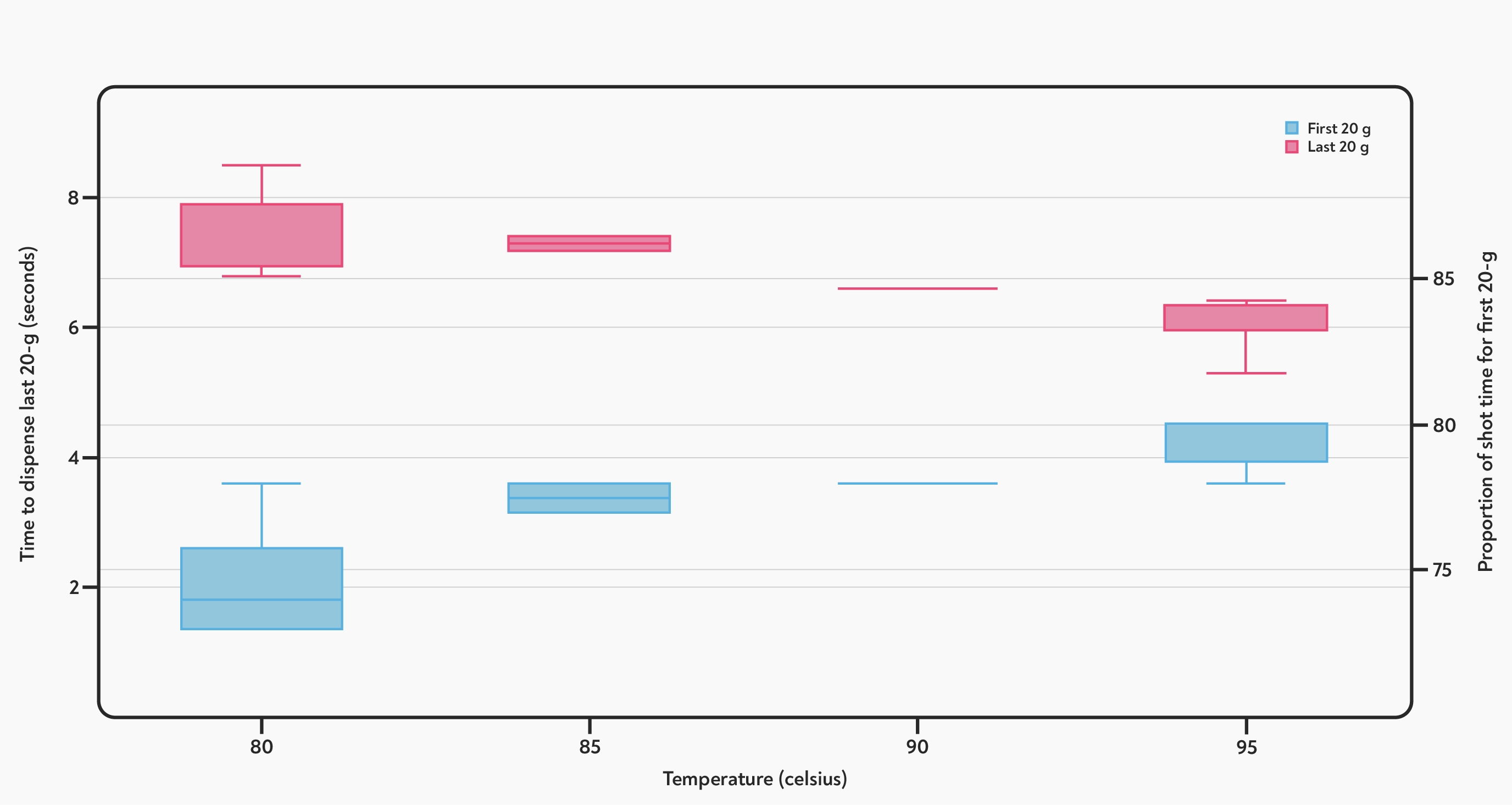 The effect of temperature on a darker roast :the red boxes show the time taken to dispense the last 20 g (left axis), and the blue boxes show the proportion of time taken to dispense the first 20 g of each shot (on the right hand axis). At higher temperatures, the first part of the shot (blue) runs more slowly, relative to the overall shot time. The second part of the shot (red) runs more quickly.
The effect of temperature on a darker roast :the red boxes show the time taken to dispense the last 20 g (left axis), and the blue boxes show the proportion of time taken to dispense the first 20 g of each shot (on the right hand axis). At higher temperatures, the first part of the shot (blue) runs more slowly, relative to the overall shot time. The second part of the shot (red) runs more quickly.
This confirmed that at higher temperatures, the first part of the shot is being slowed down, while the second part of the shot speeds up. These effects are larger than in the old coffee, and the speed up at the end is larger than in the fresh but lighter roasted coffee. This is consistent with the theory that dissolved gases are playing a part in this effect.



Do you have any publication or scientific literature I can read about the things you are talking about?
nice
Excellent post
Good post. Have a look on this also https://www.tecnora.in/blog/espresso-temperature/
I disagree with the hole hypothesis! Gas bubbles couldn’t increase resistance to the water flow rate. I wrote a whole blog post as an answer to this experiment and conclusion: https://npcoffeescience.webnode.com/l/temperatures-not-so-hidden-effect/
I’m open to discussion on this topic of course.
Thanks for taking the time to post Manasipanov3. We’ll give your piece all the consideration it deserves and add an addendum to this post to make sure we take your suggestions into account. BH
Any bubbles of gas within the puck will reduce the mass flow rate. As pressure increases at the beginning of the shot, this should develop incredibly tiny gas bubbles. They will be much smaller due to the transient effects of the increasing pressure. It’s weird (unless you’re a super-geek that loves hydrodynamics), but happens (it’s a pressure at formation vs pressure at shear crit thing if you’re interested). In addition, as they move downwards through the puck, the shear causes bubbles to form even smaller as well as increase the rate of bubble production exponentially (literally! this is not a linear function). After hitting your 9-bar, pressure is closer to static, which causes the bubbles to form larger prior to shearing and therefore will have a lesser effect on reducing mass flow rate. The gas itself has no effect on resistance, but the shear force required to move the layer of water surrounding the gas has a large effect on flow from surface tension. The critical force required to cause shearing increases as surface area decreases. Therefore, as the bubble size is smaller, the flow (out of the machine) is lower; as bubble size increases, flow increases; as bubbles stop forming altogether from the lack of CO2 left in the bean at the end of the pull, flow increases again. Flow within the puck is complicated. Suffice to say that tangential flow occurs around the bubbles, deforming them, and increases “resistance.”
On a side note, I bought my first espresso machine today! A Lelit Bianca and Atom 75. So I’m a little early to start posting anything as I won’t get my machine until next month. They were sold out of all their stock 🙁
Do you have any publication or scientific literature I can read about the things you are talking about?
Just a few considerations – viscosity of water containing gas bubbles is lower than without bubbles. There are a few publications on this topic.
As I mentioned in my post, the thing I disagree with is that gas bubbles are increasing resistance of flow. I agree that if there is more gas the mass flow will be reduced, but not because of bigger resistance! Another proof of this is from the practice where this effect could be observed and precisely measured in the HPLC system. The presence of gas in the liquid phase (water or any organic) will form gas bubbles inside of the HPLC because of the high pressure. In this situation, with the constant flow, the pressure sensor is registering pressure drop!
Thanks for this article and for Kevin N’s response. This might explain what I’m experiencing. I was trying to figure out why the flow rate didn’t seem to be affected by humidity – or rather it was affected opposite to what I understand it’s supposed to. Higher humidity, slower flow rate (unless I have this all wrong). Weirdly, the opposite seems to be happening. I don’t know how humidity affects boiler temperature and flow rate, but to my surprise, raising the temperature by 2-3 degrees on the current light and fresh roast I am using has significantly slowed down the extraction time. If Kevin or anyone is able to shed even more light on all this I’d appreciate it. Thanks!
why the Page not found?
I created a new website: http://www.npcoffeescience.com
You can find the article there.
ok,thank you~