How Does Water Dissolve Mineral Salts?
Mineral salts that are important to making coffee are known as ionic compounds, and they are held together by ionic bonds. As soon as these salts come in contact with water, they begin to dissolve. As this dissolution happens, the ions that were bonded together as a salt break apart, or dissociate. This means the ions that made up the mineral salts are now free in the water and no longer bonded to each other. This happens to all mineral salts when they dissolve.
Water is not an ion, but it acts a bit like one. Because water is a polar molecule, the slight negative and positive charge at either end of the molecule is enough to attract an ion if there are plenty of water molecules available to work together. Multiple water molecules can wrap around an ion, as illustrated below, and keep it apart from other ions.
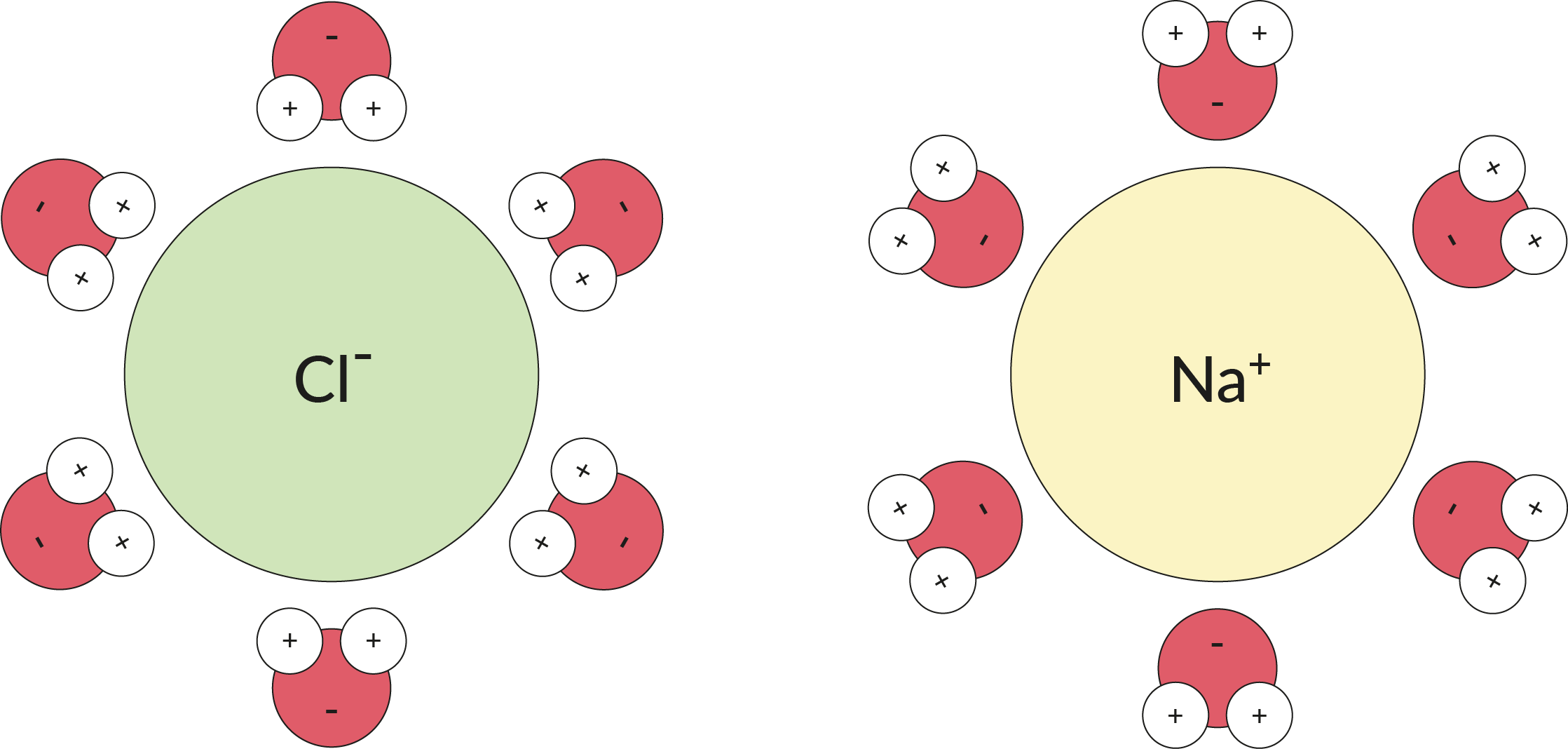
In this image you can see how table salt, NaCl, can dissociate into the chloride anion and the sodium cation after each ion is surrounded by H₂O molecules.
Stealing Electrons — Ionic Bonds
The minerals that are important to coffee all take the form of mineral salts. Mineral salts hold themselves together through ionic bonding. Instead of sharing an electron with another atom, one atom steals one or more electrons from another. This type of connection is called an ionic bond, and mineral salts are therefore known as ionic compounds.
For example, chlorine (Cl), which is used to disinfect water, has seven electrons in its outer shell. In order to become stable, it needs to ‘steal’ an electron from another atom. When it does this, it becomes negatively charged because electrons are negatively charged.
Atoms that have become charged are known as ions. The atom that steals an electron gains a negative charge and is called an anion.