What’s in The Prologue?
- This course requires some basic knowledge of chemistry. Without overloading you with information, we take you through the important chemical relationships that affect how water interacts with coffee and equipment.
- The glossary shows you how water molecules hold themselves together and explains why water is such an effective solvent.
- After exploring the chemical bonds within the water molecule, we look at how water is both able to dissolve mineral salts and gases from the atmosphere.

A photo of a paper clip floating on water
Water Facts
- Water is the only substance on Earth that occurs naturally in solid, liquid, and gas phases.
- Water molecules have a slight positive charge on one side and a slight negative charge on the other. This makes water a polar molecule.
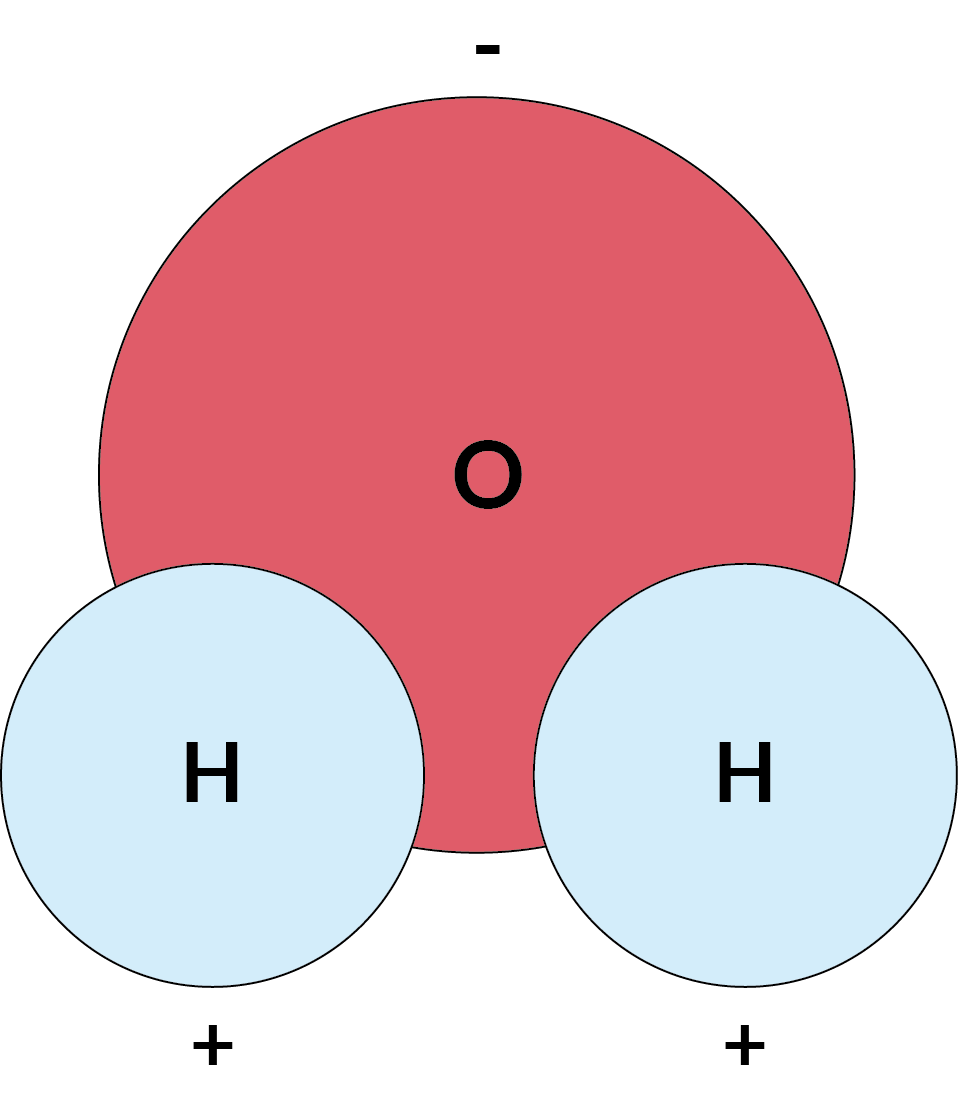
This illustration shows the opposite charges at each end of on H2O molecule
- Opposite electrical charges attract, and like electrical charges repel. The negative charge at the oxygen end of a water molecule attracts hydrogen atoms from other water molecules. It also attracts positively charged regions of other molecules. The positive hydrogen side of a water molecule attracts oxygen atoms and negatively charged regions of other molecules.
- Water is known as the universal solvent — more substances can be dissolved in water than in any other liquid.
- Water has the highest cohesion (meaning, it sticks to itself) of any nonmetallic liquid. This quality is what gives water its high surface tension — you can even float a paper clip on it if you’re careful.
- Water has relatively low adhesion with other substances (meaning, it doesn’t stick to other substances very readily). This is why it is easy to towel-dry a surface covered in water. However, water will adhere to some substances more than to others. For example, water will spread out across a glass surface, but it forms beads on top of Teflon.