As we discussed in Lesson 3.06, pressure builds up in the beans during roasting. During the roast, water in the beans evaporates to form steam, and chemical reactions produce more water vapour, carbon dioxide, and small amounts of other gases. These gases build up faster than they can escape through the bean’s pores, causing the pressure inside the beans to increase.
The pressure that builds up can change the rate of many chemical reactions that take place during roasting — speeding up many reactions, while slowing others down.
At high pressure, more molecules of gas are crammed into the same volume. Any reactions that take place when one of those gas molecules collides with another molecule will therefore happen at a faster rate, due to the higher rate of collisions.
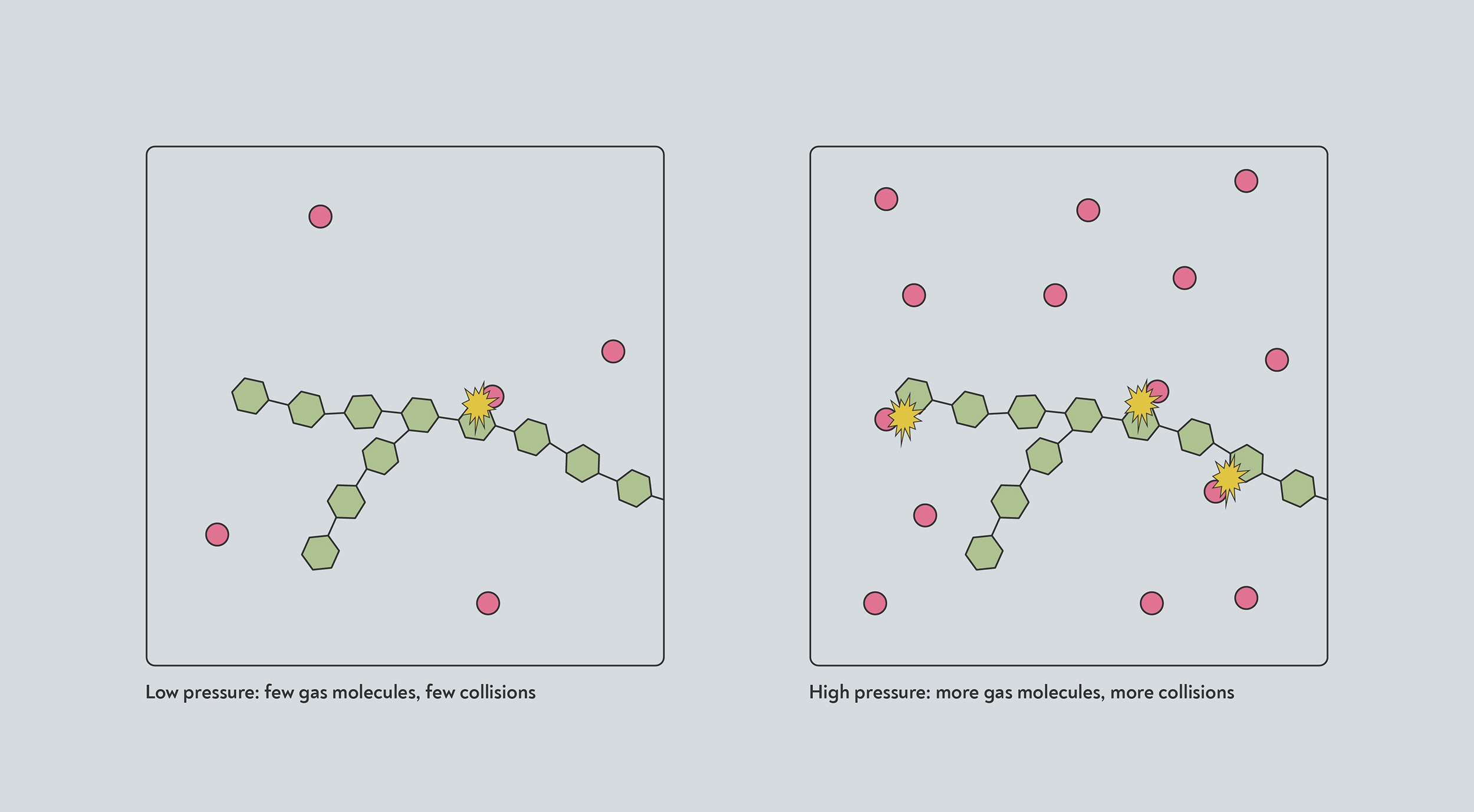 High pressure increases the rates of many reactions. At high pressure, more gas molecules (red) occupy the same amount of space. They are therefore more likely to collide with the solid (blue) and trigger a chemical reaction.
High pressure increases the rates of many reactions. At high pressure, more gas molecules (red) occupy the same amount of space. They are therefore more likely to collide with the solid (blue) and trigger a chemical reaction.
Other reactions may be slowed down by high pressure. If an intermediate that forms during a reaction has a bigger volume than the reactants, then high pressure makes it harder for that intermediate to form, therefore decreasing the reaction rate (Chen et al 2017). On the other hand, if the intermediate has a smaller volume, then high pressure can increase the rate of reaction.
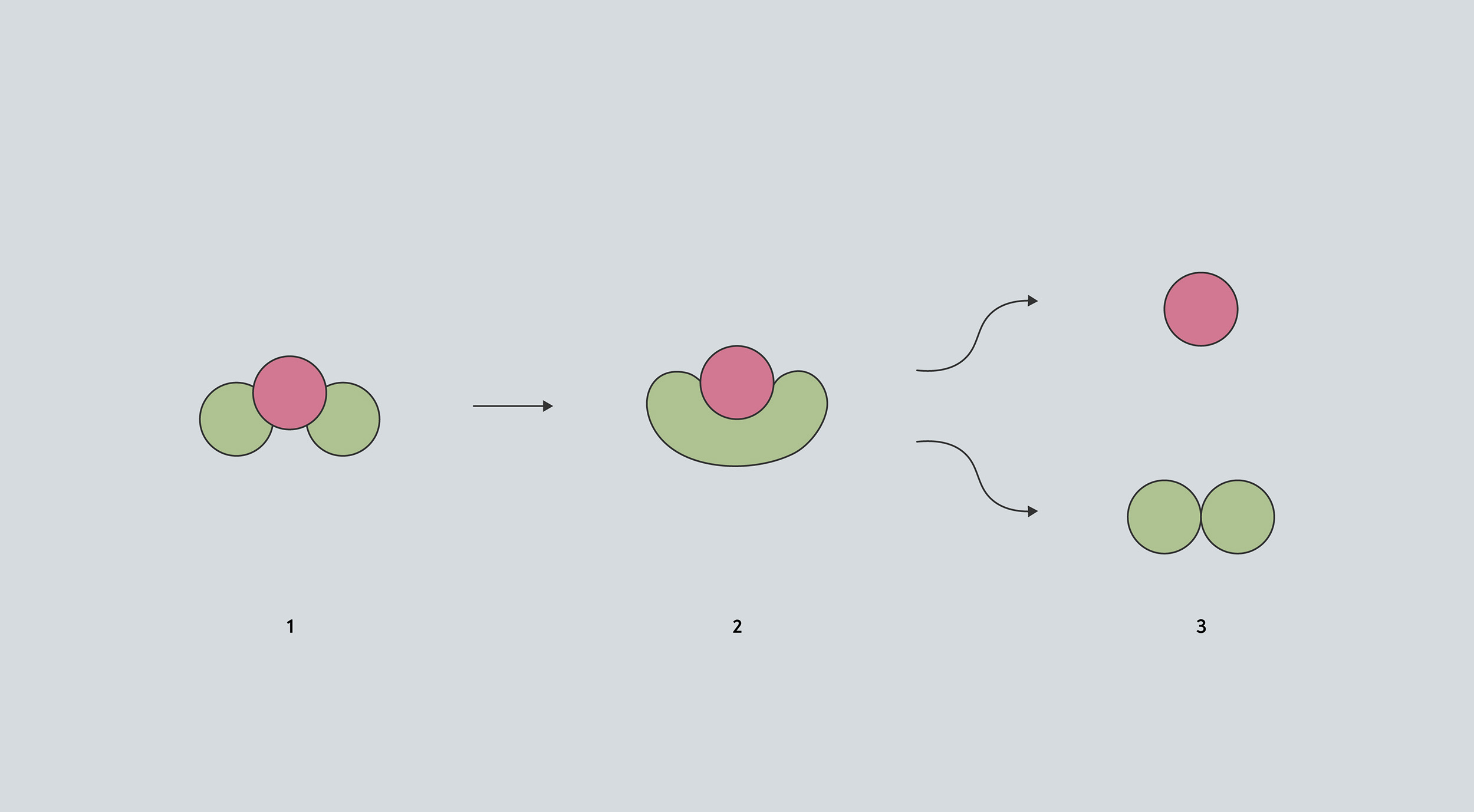 In this conceptual diagram of a reaction, the molecule (1) must pass through an intermediate stage (2) before it can break down to form other compounds (3). The intermediate stage has a bigger volume than the original molecule, so high pressure makes it harder for that intermediate to form.
In this conceptual diagram of a reaction, the molecule (1) must pass through an intermediate stage (2) before it can break down to form other compounds (3). The intermediate stage has a bigger volume than the original molecule, so high pressure makes it harder for that intermediate to form.
The important thing to understand is that pressure increases the rates of most reactions, but some reactions are inhibited by high pressure. Pressure can also tilt the balance of reactions that are in equilibrium. We will explain equilibrium reactions in Chapter 5.