The Maillard ‘reaction’ is actually a whole series of chemical reactions that are crucial to creating the characteristic flavours and brown colour of roasted coffee and many other foods – including chocolate, toast, and grilled steak. The reactions are named after Louis Camille Maillard, a French doctor who first described them in 1910.
Reducing Sugars and Amino Acids
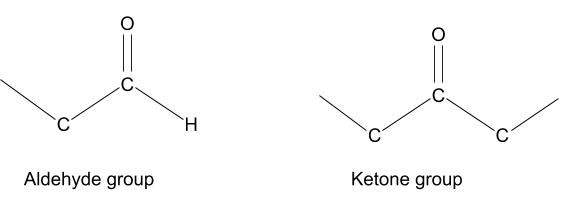
Maillard reactions occur between a reducing sugar and an amino acid. A reducing sugar is any sugar that has a free aldehyde أو ketone group. These groups contain an oxygen atom with a double bond joining it to the carbon chain, which can easily react with amino acids and many other compounds.
Amino acids are the building blocks of proteins. They have a variety of structures, but all have an amine group at one end, and a carboxyl group at the other. The amine group (NH2) on the left is the part involved in these reactions. The R, in the diagrams below, can be any of around 500 different side chains (anything from a single hydrogen atom to a chain of carbon atoms) which contributes to the complexity of these reactions.
The Reactions
When they react together, the nitrogen in the amino acid bonds to the carbon chain of the sugar, giving off one molecule of water.
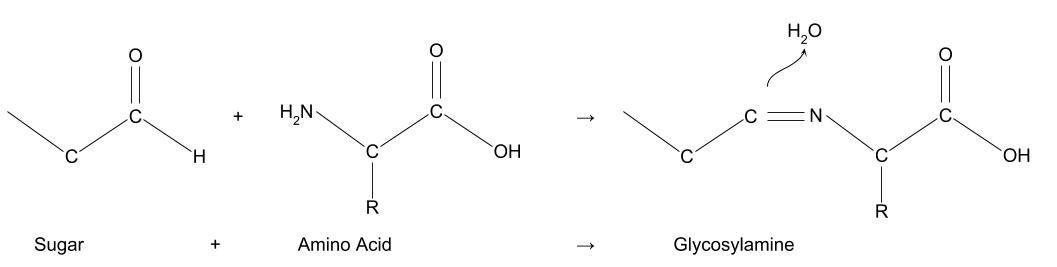
The molecule this forms (glycosylamine) is unstable, first changing its structure in a process called the Amadori rearrangement, then reacting again in one of three paths: either losing more water جزيئات to become caramel-type جزيئات, breaking down into short chain جزيئات (for example diacetyl, used to make butter-flavour popcorn), or reacting again with more amino acids.
All three products of these reactions can react again with amino acids to form the جزيئات called الميلانويدين, dark brown compounds that provide a lot of the colour in coffee and can have roasted, malty, bready, bitter, and burned flavours. الميلانويدين also play an important role in the formation of crema, by stabilising the foam (E Illy and L Navarini, 2011)
The rate of Maillard reactions becomes significant in coffee roasting from about 140° C (284° F) upwards. Above 170°C (338° F), caramelisation kicks in and starts to use up the remaining sugars. Because of the high temperature required, and because the initial reaction gives off a water molecule, the reaction is slow to get started while there is still any moisture around, which is why coffee doesn’t begin to brown in roasting until the ‘drying phase’ is complete.
The Outcome
The different possible paths these reactions can follow, combined with the range of possible amino acids and sugars involved in the reactions, means that they form a huge range of flavour compounds. The most familiar of these are the roasted, bready or bitter flavours of الميلانويدين, and the savoury flavours of peptides (think grilled meat). The reactions can also generate a wide range of smaller جزيئات as well, which can include floral, fruity and caramel odours, as well as some ‘off’ notes like oniony or earthy flavours.
The range of flavours this generates is crucial to the complexity of aromas in roasted coffee, and is part of the reason the sugar content of green beans is so important to the final flavour, even if very little sugar survives roasting intact. Quakers (unripe coffee beans) are a great demonstration of this – the lack of sugar means that Maillard and caramelisation reactions cannot take place, and the resulting beans are pale and lacking in flavour.
As well as flavour, الميلانويدين formed in the Maillard reactions also play an important role in forming and stabilising crema in espresso, and provide body to brewed coffee. They may also provide some of coffee’s health benefits – especially antioxidant and anti-inflammatory activity (AS Moreira et al, 2012).
المراجع
E Illy and L Navarini, 2011. Neglected Food Bubbles: The Espresso Coffee Foam. doi: 10.1007/s11483-011-9220-5
AS Moreira, FM Nunes, MR Domingues, MA Coimbra, 2012. Coffee الميلانويدين: structures, mechanisms of formation and potential health impacts. doi: 10.1039/c2fo30048f



Although its sad that many of our recourse comes from years 2011 and 2012 but they still can provide a good range of information for beginners and intermediate coffee roasters and people who are interested in coffee research .
Thank you