Concentration Gradient and Diffusion
The most pronounced difference in comparing the two categories of brew method is the change in concentration gradient as the rate of extraction is measured over time. If viewed graphically, the rate of extraction over time is profoundly different for the two methods.
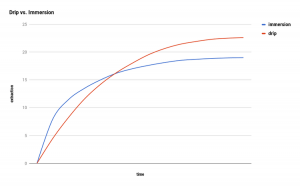
Drip vs Immersion reproduced with permission from Scott Rao
In water, coffee molecules are naturally drawn to spread out from areas of high concentration to areas of low concentration. This movement is called diffusion, with the movement occurring along what’s known as a concentration gradient.
When you put a tea bag into a cup of hot water and you don’t jiggle it about, the regions in the cup very close to the tea bag will have a higher concentration of dissolved tea solids. The inside of the tea bag has the highest concentration and the outer rim of the cup has the lowest concentration (unless you leave the bag in for a very long time). This is a concentration gradient. The gradient is steeper when the difference in concentration is bigger. In the case of a tea bag and hot water, you might think you reduce this gradient if you jiggle the tea bag about and mix it all up but this actually increases the gradient. This is because the concentration of the liquid closest to the tea leaves becomes less concentrated after jiggling the tea bag — it mixes with all that weak tea at the edges of the cup. This increase in the concentration gradient will promote a higher rate of diffusion and thus, a higher rate of extraction. This helps explain why agitation increases the rate of extraction, e.g. when you break the crust in a cupping bowl. If you don’t jiggle the tea bag, you know the tea will eventually spread itself out, but this is a slow process, slowed further still by the gradual drop in temperature of the brew water.
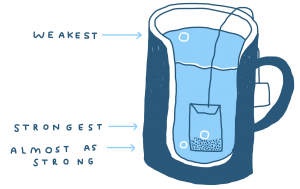
This image depicts the concentration gradient between the strong liquid in the tea bag,